Abstract
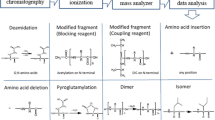
A liquid chromatography-high resolution mass spectrometry (LC-HRMS) method was developed using three peptide drugs: salmon calcitonin, bivalirudin, and exenatide as model systems to assess the suitability of this approach for monitoring peptide drug product quality. Calcitonin and its related impurities displayed linear responses over the range from 0.1 to 10 μM (R 2 values for calcitonin salmon, Glu14-calcitonin, and acetyl-calcitonin were 0.995, 0.996, and 0.993, respectively). Intra-assay precision in terms of relative standard deviation (%RSD) was less than 10% at all tested concentrations. The accuracy of the method was greater than 85% as measured by spiking 0.1, 0.3, and 1% of Glu14-calcitonin and acetyl-calcitonin into a stock calcitonin solution. Limits of detection for calcitonin, Glu14-calcitonin, and acetyl-calcitonin were 0.02, 0.03, and 0.04 μM, respectively, indicating that an impurity present at less than 0.1% (0.1 μM) of the drug product API concentration (107 μM) could be detected. Method validation studies analyzing bivalirudin and exenatide drug products exhibited similar results to calcitonin salmon in regard to high selectivity, sensitivity, precision, and linearity. Added benefits of using LC-HRMS-based methods are the ability to also determine amino acid composition, confirm peptide sequence, and quantify impurities, even when they are co-eluting, within a single experiment. LC-HRMS represents a promising approach for the quality control of peptides including the measurement of any peptide-related impurities. While the development work performed here is focus on peptide drug products, the principles could be adapted to peptide drug substance.




Similar content being viewed by others
Abbreviations
- AA:
-
Amino acid
- AC:
-
N-acetyl-calcitonin salmon
- API:
-
Active pharmaceutical ingredient
- BA:
-
Beta-aspartic acid-bivalirudin
- BV:
-
Bivalirudin
- BV[12–20]:
-
12–20 fragment of bivalirudin
- sCT:
-
Calcitonin salmon
- DCM:
-
Dichloromethane
- DG:
-
Des-glycine-bivalirudin
- EIC:
-
Extracted ion chromatogram
- ET:
-
Exenatide
- Glu14 :
-
Glu14-calcitonin salmon
- Glu20 :
-
Glu20-calcitonin salmon
- LC-MS:
-
Liquid chromatography mass spectrometry
- LC-HRMS:
-
Liquid chromatography-high resolution mass spectrometry
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- MEW:
-
Mass extraction window
- MS/MS:
-
Tandem mass spectrometry
- NDA/ANDA:
-
New Drug Application/Abbreviated New Drug Application
- NMT:
-
Not more than
- PG:
-
Plus-glycine-bivalirudin
- SPPS:
-
Solid-phase peptide synthesis
- TIC:
-
Total ion chromatogram
References
Ermerand J, Vogel M. Applications of hyphenated LC-MS techniques in pharmaceutical analysis. Biomed Chromatogr. 2000;14:373–83.
Kasparand AA, Reichert JM. Future directions for peptide therapeutics development. Drug Discov Today. 2013;18:807–17.
Dorpe VS, Verbeken M, Wynendaele E, De Spiegeleer B. Purity profiling of peptide drugs. J Bioanal Biomed. 2011;15:1–15.
Gucinskiand AC, Boyne 2nd MT. Identification of site-specific heterogeneity in peptide drugs using intact mass spectrometry with electron transfer dissociation. Rapid Commun Mass Spectrom: RCMS. 2014;28:1757–63.
Ramanathan R, Jemal M, Ramagiri S, Xia YQ, Humpreys WG, Olah T, et al. It is time for a paradigm shift in drug discovery bioanalysis: from SRM to HRMS. J Mass Spectrom: JMS. 2011;46:595–601.
Ramanathan R, Korfmacher W. The emergence of high-resolution MS as the premier analytical tool in the pharmaceutical bioanalysis arena. Bioanalysis. 2012;4:467–9.
Morin LP, Mess JN, Garofolo F. Large-molecule quantification: sensitivity and selectivity head-to-head comparison of triple quadrupole with Q-TOF. Bioanalysis. 2013;5:1181–93.
Wei H, Tymiak AA, Chen G. High-resolution MS for structural characterization of protein therapeutics: advances and future directions. Bioanalysis. 2013;5:1299–313.
Fung EN, Jemal M, Aubry AF. High-resolution MS in regulated bioanalysis: where are we now and where do we go from here? Bioanalysis. 2013;5:1277–84.
Dillenand L, Cuyckens F. High-resolution MS: first choice for peptide quantification? Bioanalysis. 2013;5:1145–8.
Ermerand J, Kibat P-G. A quality concept for impurities during drug development—use of the hyphenated LC–MS technique. Pharm Sci Technol Today. 1998;1:76–82.
Ermer J. The use of hyphenated LC–MS technique for characterisation of impurity profiles during drug development. J Pharm Biomed Anal. 1998;18:707–14.
Chopra S, Pendela M, Hoogmartens J, Van Schepdael A, Adams E. Impurity profiling of capreomycin using dual liquid chromatography coupled to mass spectrometry. Talanta. 2012;100:113–22.
Chopra S, Van Schepdael A, Hoogmartens J, Adams E. Characterization of impurities in tylosin using dual liquid chromatography combined with ion trap mass spectrometry. Talanta. 2013;106:29–38.
Gucinskiand AC, Boyne 2nd MT. Evaluation of intact mass spectrometry for the quantitative analysis of protein therapeutics. Anal Chem. 2012;84:8045–51.
Chesnut 3rd CH, Azria M, Silverman S, Engelhardt M, Olson M, Mindeholm L. Salmon calcitonin: a review of current and future therapeutic indications. Osteoporos Int. 2008;19:479–91.
USP 36-NF 31 (United States Pharmacopeia Convention). Calcitonin salmon, calcitonin salmon injection, and calcitonin nasal solution. USP;2013. p. 2736-2741.
Reedand MD, Bell D. Clinical pharmacology of bivalirudin. Pharmacotherapy. 2002;22:105S–11S.
Cvetkovicand RS, Plosker GL. Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea). Drugs. 2007;67:935–54.
Liu B, Dong Q, Shi L, Wang M, Li C, Wu Y, et al. Development and validation of a reverse-phase high performance liquid chromatography method for determination of exenatide in poly(lactic-co-glycolic acid) microspheres. Chem Res Chin Univ. 2010;26:33–7.
Acknowledgments
Internal funding for this work was provided by the CDER Critical Path Program and the Office of Generic Drugs (MTB).
Disclaimer
The findings and conclusions of this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any agency determination or policy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, K., Geerlof-Vidavisky, I., Gucinski, A. et al. Liquid Chromatography-High Resolution Mass Spectrometry for Peptide Drug Quality Control. AAPS J 17, 643–651 (2015). https://doi.org/10.1208/s12248-015-9730-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-015-9730-z