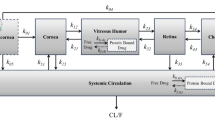
Abstract
Asialo, tri-antennary oligosaccharide (NA3 glycan) is an endogenous compound, which supports proper folding of outer segment membranes, promotes normal ultrastructure, and maintains protein expression patterns of photoreceptors and Müller cells in the absence of retinal pigment epithelium support. It is a potential new therapeutic for atrophic age-related macular degeneration (AMD) and other retinal degenerative disorders. Herein, we evaluate the safety, in vitro stability, ocular pharmacokinetics and biodistribution of NA3. NA3 was injected into the vitreous of New Zealand white rabbits at two concentrations viz. 1 nM (minimum effective concentration (MEC)) and 100 nM (100XMEC) at three time points. Safety was evaluated using routine clinical and laboratory tests. Ocular pharmacokinetics and biodistribution of [3H]NA3 were estimated using scintillation counting in various parts of the eye, multiple peripheral organs, and plasma. Pharmacokinetic parameters were estimated by non-compartmental modeling. A 2-aminobenzamide labeling and hydrophilic interaction liquid interaction chromatography were used to assess plasma and vitreous stability. NA3 was well tolerated by the eye. The concentration of NA3 in eye tissues was in the order: vitreous > retina > sclera/choroid > aqueous humor > cornea > lens. Area under the curve (0 to infinity) (AUC∞) was the highest in the vitreous thereby providing a positive concentration gradient for NA3 to reach the retina. Half-lives in critical eye tissues ranged between 40 and 60 h. NA3 concentrations were negligible in peripheral organs. Radioactivity from [3H]NA3 was excreted via urine and feces. NA3 was stable at 37°C in vitreous over a minimum of 6 days, while it degraded rapidly in plasma. Collectively, these results document that NA3 shows a good safety profile and favorable ocular pharmacokinetics.









Similar content being viewed by others
References
Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60.
Zhang K, Zhang L, Weinreb RN. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat Rev. 2012;11:541–59.
Haddad S, Chen CA, Santangelo SL, Seddon JM. The genetics of age-related macular degeneration: a review of progress to date. Surv Ophthalmol. 2006;51:316–63.
Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev Neurosci. 2006;7:860–72.
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317.
Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32.
Kaplan HJ, Leibole MA, Tezel T, Ferguson TA. Fas ligand (CD95 ligand) controls angiogenesis beneath the retina. Nat Med. 1999;5:292–7.
Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43.
Krzystolik MG, Afshari MA, Adamis AP, Gaudreault J, Gragoudas ES, Michaud NA, et al. Prevention of experimental choroidal neovascularization with intravitreal anti-vascular endothelial growth factor antibody fragment. Arch Ophthalmol. 2002;120:338–46.
Okamoto N, Tobe T, Hackett SF, Ozaki H, Vinores MA, LaRochelle W, et al. Transgenic mice with increased expression of vascular endothelial growth factor in the retina: a new model of intraretinal and subretinal neovascularization. Am J Pathol. 1997;151:281–91.
Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76.
Vingerling JR, Dielemans I, Hofman A, Grobbee DE, Hijmering M, Kramer CF, et al. The prevalence of age-related maculopathy in the Rotterdam study. Ophthalmology. 1995;102:205–10.
Friedman E. The pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2008;146(3):348–9.
Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72.
Damico FM, Gasparin F, Scolari MR, Pedral LS, Takahashi BS. New approaches and potential treatments for dry age-related macular degeneration. Arq Bras Oftalmol. 2012;75(1):71–5.
Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, et al. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci. 1999;40(3):775–9.
Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, et al. Glycation-altered proteolysis as a pathobiological mechanism that links dietary glycemic index, aging, and age-related disease (in nondiabetics). Aging Cell. 2012;11:1–13.
Weikel KA, Fitzgerald P, Shang F, Caceres MA, Bian Q, Handa JT, et al. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest Ophthalmol Vis Sci. 2012;53:622–32.
Palamoor M, Jablonski MM. Poly(ortho ester) nanoparticle-based targeted intraocular therapy for controlled release of hydrophilic molecules. Mol Pharm. 2013;10(2):701–8.
Swaminathan S, Jablonski MM. Non-biological membranes as drug delivery systems. J Memb Sci Technol. 2012;2(3):e108.
Swaminathan S, Vavia P, Trotta F, Cavalli R. Nanosponges encapsulating dexamethasone for ocular delivery: formulation design, physicochemical characterization, safety and corneal permeability assessment. J Biomed Nanotechnol. 2013;9(6):998–1007.
Jablonski MM, inventor; The University of Tennessee Research Foundation, assignee. Glycan binding proteins as therapeutic targets for retinal disorders and treatment methods based thereon. USA patent 8092825. 2012 Jan 10, 2012.
Jablonski M, Ervin C. A closer look at lactose mediated support of retinal morphogenesis. Anat Rec. 2000;259:205–14.
Jablonski MM, Iannaccone A. Lactose supports Muller cell protein expression patterns in the absence of the retinal pigment epithelium. Mol Vis. 2001;7:27–35.
Jablonski MM, Wohabrebbi A. Lactose promotes organized photoreceptor outer segment assembly and preserves expression of photoreceptor proteins in retinal degeneration. Mol Vis. 1999;5:16.
Fukuda M. Chemical labeling of carbohydrates by oxidation and sodium borohydride reduction. Curr Protoc Mol Biol. 1994;17.5.1–17.5.8:Supplement 26.
Muraoka Y, Ikeda H, Nakano N, Hangai M, Toda Y, Okamoto-Furuta K, et al. Real-time imaging of rabbit retina with retinal degeneration by using spectral-domain optical coherence tomography. PLoS One. 2012;7(4):e36135.
Skapek S, Lin S, Jablonski M, McKeller R, Tan M, Hu N, et al. Persistent expression of cyclin D1 disrupts normal photoreceptor differentiation and retina development. Oncogene. 2001;20:6742–51.
Bulletin PT. DeadEnd™ Fluorometric TUNEL System: instructions for use of product G3250. Madison, WI, USA.
Kim H, Csaky KG, Chan CC, Bungay PM, Lutz RJ, Dedrick RL, et al. The pharmacokinetics of rituximab following an intravitreal injection. Exp Eye Res. 2006;82(5):760–6.
Kim H, Csaky KG, Gravlin L, Yuan P, Lutz RJ, Bungay PM, et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26(5):523–30.
López–Cortés L, Pastor–Ramos M, Ruiz–Valderas R, Cordero E, Uceda–Montañés A, Claro–Cala C, et al. Intravitreal pharmacokinetics and retinal concentrations of ganciclovir and foscarnet after intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2001;42(5):1024–8.
Sinapis CI, Routsias JG, Sinapis AI, Sinapis DI, Agrogiannis GA, Pantopoulou A, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin®) in rabbits. Clin Ophthalmol. 2011;5:697–704.
Shahar J, Zemel E, Perlman I, Loewenstein A. Physiological and toxicological effects of cefuroxime on the albino rabbit retina. Invest Ophthalmol Vis Sci. 2012;53(2):906–14.
Wang XF, Iannaccone A, Jablonski MM. Permissive glycan support of photoreceptor outer segment assembly occurs via a non-metabolic mechanism. Mol Vis. 2003;9:701–9.
Stiemke MM, Hollyfield JG. Effect of sugars on photoreceptor outer segment assembly. In: Anderson RE, Hollyfield JG, LaVail MM, editors. Degenerative diseases of the retina. New York: Plenum; 1995. p. 129–37.
Stiemke MM, Landers RA, Al-Ubaidi MR, Hollyfield JG. Photoreceptor outer segment development in Xenopus laevis: influence of the pigment epithelium. Dev Biol. 1994;162:169–80.
Varner HH, Rayborn ME, Osterfeld AM, Hollyfield JG. Localization of proteoglycan within the matrix sheath of cone photoreceptors. Exp Eye Res. 1987;44:633–42.
Ganesh S, Chintala S. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS ONE. 2011;6(3):e18305.
Sarthy V. Focus on molecules: glial fibrillary acidic protein (GFAP). Exp Eye Res. 2007;84:381–2.
Erickson PA, Fisher SK, Guerin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Muller cells after retinal detachment. Exp Eye Res. 1987;44:37–48.
Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res. 1995;61:83–90.
Woldemussie E, Wijono M, Ruiz G. Muller cell response to laser-induced in intraocular pressure in rats. Glia. 2004;47:109–19.
Kerr et al. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239.
Darzynkiewicz et al. Analysis of apoptosis by cytometry using TUNEL assay. Methods Enzymol. 2008;44(3):250.
Barber A, Lieth E, Khin S, Antonetti D, Buchanan A, Gardner T. Neural apoptosis in the retina during experimental and human diabetes: early onset and effect of insulin. J Clin Investig. 1998;102(4):783–91.
Kadam RS, Jadhav G, Ogidigben M, Kompella UB. Ocular pharmacokinetics of dorzolamide and brinzolamide after single and multiple topical dosing: implications for effects on ocular blood flow. Drug Metab Dispos. 2011;39(9):1529–37.
Wu Z, Sadda SR. Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case report. Ann Acad Med Singap. 2008;37(7):591–3.
Laude A, Tan L, Wilson C, Lascaratos G, Elashry M, Aslam T, et al. Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Prog Retin Eye Res. 2010;29:466–75.
Acknowledgments
The research work was supported by the following grants: March of Dimes, University of Tennessee Research Foundation, International Retinal Research Foundation, an unrestricted grant from Research to Prevent Blindness, Knights Templar Eye Foundation, and Fight for Sight. The authors would like to thank Bob M. Moore, Ph.D. and Vivian S. Loveless, Pharm. D. (Department of Pharmaceutical Sciences, UTHSC) for providing their expertise in radiolabeling and scintillation counting.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
a Blank plasma chromatogram, b chromatogram of 2-AB-NA3 glycan, c chromatogram of plasma spiked with 2-AB-NA3 glycan, and d chromatogram of vitreous spiked with 2-AB-NA3 glycan. (JPEG 57 kb)
Supplementary Table 1
Gradient program for estimation of 2-AB NA3 using HILIC. (DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Swaminathan, S., Li, H., Palamoor, M. et al. Novel Endogenous Glycan Therapy for Retinal Diseases: Safety, In Vitro Stability, Ocular Pharmacokinetic Modeling, and Biodistribution. AAPS J 16, 311–323 (2014). https://doi.org/10.1208/s12248-014-9563-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-014-9563-1