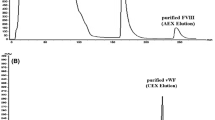
Abstract
Factor VIII (FVIII) is a multi-domain glycoprotein that is an essential cofactor in the blood coagulation cascade. Its deficiency or dysfunction causes hemophilia A, a bleeding disorder. Replacement using exogenous recombinant human factor VIII (rFVIII) is the first line of therapy for hemophilia A. The role of glycosylation on the activity, stability, protein–lipid interaction, and immunogenicity of FVIII is not known. In order to investigate the role of glycosylation, a deglycosylated form of FVIII was generated by enzymatic cleavage of carbohydrate chains. The biochemical properties of fully glycosylated and completely deglycosylated forms of rFVIII (degly rFVIII) were compared using enzyme-linked immunosorbent assay, size exclusion chromatography, and clotting activity studies. The biological activity of degly FVIII decreased in comparison to the fully glycosylated protein. The ability of degly rFVIII to interact with phosphatidylserine containing membranes was partly impaired. Data suggested that glycosylation significantly influences the stability and the biologically relevant macromolecular interactions of FVIII. The effect of glycosylation on immunogenicity was investigated in a murine model of hemophilia A. Studies showed that deletion of glycosylation did not increase immunogenicity.




Similar content being viewed by others
Abbreviations
- aPTT:
-
Activated partial thromboplastin time
- BPS:
-
Brain phosphatidylserine
- BSA:
-
Bovine serum albumin
- CHO:
-
Chinese hamster ovarian cell line
- DEA:
-
Diethanolamine buffer
- Degly FVIII:
-
Deglycosylated recombinant human factor VIII
- DMPC:
-
Dimyristoylphosphatidylcholine
- ELISA:
-
Enzyme-linked immunosorbent assay
- Endo:
-
Endoglycosidase
- FVIII:
-
Factor VIII
- PB:
-
Phosphate buffer
- PBA:
-
Phosphate buffer with 1% bovine serum albumin
- PBT:
-
Tween 20 containing phosphate buffer
- p-NPP:
-
p-nitrophenylphosphate
- PS:
-
Phospatidylserine
- rFVIII:
-
Recombinant human factor VIII
- SEC:
-
Size exclusion chromatography
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
References
Kaufman RJ. Biological regulation of factor VIII activity. Annu Rev Med. 1992;43:325–39.
Pittman DD, Tomkinson KN, Kaufman RJ. Post-translational requirements for functional factor V and factor VIII secretion in mammalian cells. J Biol Chem. 1994;269(25):17329–37.
Kaufman RJ, Wasley LC, Dorner AJ. Synthesis, processing, and secretion of recombinant human factor VIII expressed in mammalian cells. J Biol Chem. 1988;263(13):6352–62.
Bovenschen N, Rijken DC, Havekes LM, van Vlijmen BJ, Mertens K. The B domain of coagulation factor VIII interacts with the asialoglycoprotein receptor. J Thromb Haemost. 2005;3(6):1257–65.
Medzihradszky KF, Besman MJ, Burlingame AL. Structural characterization of site-specific N-glycosylation of recombinant human factor VIII by reversed-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal Chem. 1997;69(19):3986–94.
Medzihradszky KF, Besman MJ, Burlingame AL. Reverse-phase capillary high performance liquid chromatography/high performance electrospray ionization mass spectrometry: an essential tool for the characterization of complex glycoprotein digests. Rapid Commun Mass Spectrom. 1998;12(8):472–8.
Saenko EL, Scandella D, Yakhyaev AV, Greco NJ. Activation of factor VIII by thrombin increases its affinity for binding to synthetic phospholipid membranes and activated platelets. J Biol Chem. 1998;273(43):27918–26.
Lollar P. Molecular characterization of the immune response to factor VIII. Vox Sang. 2002;83(Suppl 1):403–8.
Saenko EL, Ananyeva NM, Kouiavskaia DV, Khrenov AV, Anderson JA, Shima M, et al. Haemophilia A: effects of inhibitory antibodies on factor VIII functional interactions and approaches to prevent their action. Haemophilia. 2002;8(1):1–11.
Medzihradszky KF. Characterization of protein N-glycosylation. Methods Enzymol. 2005;405:116–38.
Narhi LO, Arakawa T, Aoki KH, Elmore R, Rohde MF, Boone T, et al. The effect of carbohydrate on the structure and stability of erythropoietin. J Biol Chem. 1991;266(34):23022–6.
Helenius A. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol Biol Cell. 1994;5(3):253–65.
Herrmann JM, Malkus P, Schekman R. Out of the ER—outfitters, escorts and guides. Trends Cell Biol. 1999;9(1):5–7.
Haraguchi M, Yamashiro S, Furukawa K, Takamiya K, Shiku H. The effects of the site-directed removal of N-glycosylation sites from beta-1,4-N-acetylgalactosaminyltransferase on its function. Biochem J. 1995;312(Pt 1):273–80.
Lige B, Ma S, van Huystee RB. The effects of the site-directed removal of N-glycosylation from cationic peanut peroxidase on its function. Arch Biochem Biophys. 2001;386(1):17–24.
van Hoek AN, Wiener MC, Verbavatz JM, Brown D, Lipniunas PH, Townsend RR, et al. Purification and structure-function analysis of native, PNGase F-treated, and endo-beta-galactosidase-treated CHIP28 water channels. Biochemistry. 1995;34(7):2212–9.
Mitra N, Sharon N, Surolia A. Role of N-linked glycan in the unfolding pathway of erythrina corallodendron lectin. Biochemistry. 2003;42(42):12208–16.
Joao HC, Dwek RA. Effects of glycosylation on protein structure and dynamics in ribonuclease B and some of its individual glycoforms. Eur J Biochem. 1993;218(1):239–44.
Diaz CL, Logman T, Stam HC, Kijne JW. Sugar-binding activity of pea lectin expressed in white clover hairy roots. Plant Physiol. 1995;109(4):1167–77.
Mouricout M. Interactions between the enteric pathogen and the host. An assortment of bacterial lectins and a set of glycoconjugate receptors. Adv Exp Med Biol. 1997;412:109–23.
Sharon N, Lis H. Lectins as cell recognition molecules. Science. 1989;246(4927):227–34.
Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, et al. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res. 1998;15(4):641–9.
Utsumi J, Yamazaki S, Hosoi K, Shimizu H, Kawaguchi K, Inagaki F. Conformations of fibroblast and E. coli-derived recombinant human interferon-beta s as studied by nuclear magnetic resonance and circular dichroism. J Biochem. 1986;99(5):1533–5.
Elder B, Lakich D, Gitschier J. Sequence of the murine factor VIII cDNA. Genomics. 1993;16(2):374–9.
Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost. 2000;84(5):826–32.
Tarentino AL, Plummer TH Jr. Substrate specificity of Flavobacterium meningosepticum Endo F2 and endo F3: purity is the name of the game. Glycobiology. 1994;4(6):771–3.
Trimble RB, Tarentino AL. Identification of distinct endoglycosidase (endo) activities in flavobacterium meningosepticum: endo F1, endo F2, and endo F3. Endo F1 and endo H hydrolyze only high mannose and hybrid glycans. J Biol Chem. 1991;266(3):1646–51.
Over J. Methodology of the one-stage assay of Factor VIII (VIII:C). Scand J Haematol Suppl. 1984;41:13–24.
Ramani K, Balasubramanian SV. Fluorescence properties of Laurdan in cochleate phases. Biochim Biophys Acta. 2003;1618(1):67–78.
Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234(3):466–8.
Purohit VS, Ramani K, Kashi RS, Durrani MJ, Kreiger TJ, Balasubramanian SV. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochim Biophys Acta. 2003;1617(1–2):31–8.
Ramani K, Purohit V, Middaugh CR, Balasubramanian SV. Aggregation kinetics of recombinant human FVIII (rFVIII). J Pharm Sci. 2005;94(9):2023–9.
Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII:C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73(2):247–51.
Griffin BD, Micklem LR, McCann MC, James K, Pepper DS. The production and characterisation of a panel of ten murine monoclonal antibodies to human procoagulant factor VIII. Thromb Haemost. 1986;55(1):40–6.
Grillo AO, Edwards KL, Kashi RS, Shipley KM, Hu L, Besman MJ, et al. Conformational origin of the aggregation of recombinant human factor VIII. Biochemistry. 2001;40(2):586–95.
Qian J, Borovok M, Bi L, Kazazian HH Jr, Hoyer LW. Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost. 1999;81(2):240–4.
Shipley JM, Grubb JH, Sly WS. The role of glycosylation and phosphorylation in the expression of active human beta-glucuronidase. J Biol Chem. 1993;268(16):12193–8.
Ioannou YA, Zeidner KM, Grace ME, Desnick RJ. Human alpha-galactosidase A: glycosylation site 3 is essential for enzyme solubility. Biochem J. 1998;332(Pt 3):789–97.
Di Natale P, Vanacore B, Daniele A, Esposito S. Heparan N-sulfatase: in vitro mutagenesis of potential N-glycosylation sites. Biochem Biophys Res Commun. 2001;280(5):1251–7.
Purohit S, Shao K, Balasubramanian SV, Bahl OP. Mutants of human choriogonadotropin lacking N-glycosyl chains in the alpha-subunit. 1. Mechanism for the differential action of the N-linked carbohydrates. Biochemistry. 1997;36(40):12355–63.
Gilbert GE, Drinkwater D. Specific membrane binding of factor VIII is mediated by O-phospho-l-serine, a moiety of phosphatidylserine. Biochemistry. 1993;32(37):9577–85.
Ramani K, Purohit VS, Miclea RD, Middaugh CR, Balasubramanian SV. Lipid binding region (2303–2332) is involved in aggregation of recombinant human FVIII (rFVIII). J Pharm Sci. 2005;94(6):1288–99.
Weiss WFt, Young TM, Roberts CJ. Principles, approaches, and challenges for predicting protein aggregation rates and shelf life. J Pharm Sci. 2009;98(4):1246–77.
Andrews JM, Roberts CJ. Non-native aggregation of alpha-chymotrypsinogen occurs through nucleation and growth with competing nucleus sizes and negative activation energies. Biochemistry. 2007;46(25):7558–71.
Patten PA, Schellekens H. The immunogenicity of biopharmaceuticals. Lessons learned and consequences for protein drug development. Dev Biol (Basel). 2003;112:81–97.
Purohit VS, Middaugh CR, Balasubramanian SV. Influence of aggregation on immunogenicity of recombinant human factor VIII in hemophilia A mice. J Pharm Sci. 2006;95(2):358–71.
Shen BW, Spiegel PC, Chang CH, Huh JW, Lee JS, Kim J, et al. The tertiary structure and domain organization of coagulation factor VIII. Blood. 2008;111(3):1240–7.
Acknowledgements
The authors thank the Pharmaceutical Sciences Instrumentation Facility, University at Buffalo (UB), for the use of the Circular Dichroism and the Fluorescence spectrophotometers. We thank the Hemophilia Center of Western New York for providing rFVIII. We express gratitude to Dr. Robert Straubinger (UB) for suggestions and review of this manuscript. This work was supported by NHLBI, National Institute of Health grant R01 HL-70227 to SVB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Matthew P. Kosloski and Razvan D. Miclea equally contributed to the manuscript.
Rights and permissions
About this article
Cite this article
Kosloski, M.P., Miclea, R.D. & Balu-Iyer, S.V. Role of Glycosylation in Conformational Stability, Activity, Macromolecular Interaction and Immunogenicity of Recombinant Human Factor VIII. AAPS J 11, 424–431 (2009). https://doi.org/10.1208/s12248-009-9119-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9119-y