Abstract
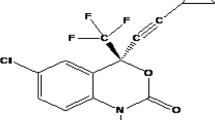
The highly potent anti-HIV agent UC781 is being evaluated for use in topical microbicides to prevent HIV transmission. However, UC781 is extremely hydrophobic with poor water solubility, a property that may complicate appropriate formulation of the drug. In this study, we examined the ability of several cyclodextrins, beta-cyclodextrin (βCD), methyl-beta-cyclodextrin (MβCD), and 2-hydroxylpropyl-beta-cyclodextrin (HPβCD), to enhance the aqueous solubility of UC781. Each of the cyclodextrins provided dramatic increases in UC781 aqueous solubility, the order being MβCD>HPβCD>βCD. The complexation constants (K 1:1) of the inclusion complexes were determined via a phase solubility technique using high-performance liquid chromatography and showed that UC781 solubility increased linearly as a function of cyclodextrin concentration. Ultraviolet spectroscopy, Fourier transform infrared spectroscopy, differential scanning calorimetry, and 2D 1H ROESY NMR spectroscopy were used to further characterize these UC781/cyclodextrin complexes. The inhibitory potency of UC781 and its HPβCD inclusion complex were evaluated using an in vitro HIV-1 reverse transcriptase inhibition assay The inhibitory potency of the UC781/HPβCD complex was 30-fold greater than that of UC781 alone, showing that the complexed drug is able to provide substantial inhibition of its target. The enhancement of UC781 aqueous solubility is essential for the development of a useful vaginal microbicide dosage form, and our data suggest that UC781/cyclodextrin inclusion complexes may be useful in this context.







Similar content being viewed by others
References
J. Balzarini, L. Naesens E. Verbeken, et al. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 12:1129–1138 (1998).
J. Balzarini, H. Pelemans S. Aquaro, et al. Highly favorable antiviral activity and resistance profile of the novel thiocarboxanilide pentenyloxy ether derivatives UC-781 and UC-82 as inhibitors of human immunodeficiency virus type 1 replication. Mol. Pharmacol. 50(2):394–401 (1996).
J. Barnard, G. Borkow, and M. A. Parniak. The thiocarboxanilide nonnucleoside UC781 is a tight-binding inhibitor of HIV-1 reverse transcriptase. Biochemistry 36(25):7786–7792 (1997).
J. P. Bader, J. B. McMahon R. J. Schultz, et al. Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc. Natl. Acad. Sci. USA 88(15):6740–6744 (1991), 1991 Aug1.
G. Borkow, D. Arion, M. A. Wainberg, and M. A. Parniak. The Thiocarboxanilide Nonnucleoside Inhibitor UC781 Restores Antiviral Activity of 39-Azido-39-Deoxythymidine (AZT) against AZT-Resistant Human Immunodeficiency Virus Type 1. Antimicrob. Agents Chemother. 43(2):259–263 (1999).
W. Robert, J. Buckheit, M. J. Snow V. Fliakas-Boltz, et al. Highly Potent Oxathiin Carboxanilide Derivatives with Efficacy against Nonnucleoside Reverse Transcriptase Inhibitor-Resistant Human Immunodeficiency Virus Isolates. Antimicrob. Agents Chemother. 41(4):831–837 (1997).
G. Borkow, J. Barnard, T. M. Nguyen, A. A. Belmonte, M. A. Wainberg, and M. A. Parniak. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71(4):3023–3030 (1997).
S. Liu, H. Lu, A. R. Neurath, and S. Jiang. Combination of candidate microbicides cellulose acetate 1,2-benzenedicarboxylate and UC781 has synergistic and complementary effects against human immunodeficiency virus type 1 infection. Antimicrob. Agents Chemother. 49(5):1830–1836 (2005).
S. Deferme, J. V. Gelder F. Ingels, et al. Intestinal absorption characteristics of the low solubility thiocarboxanilide UC-781. Int. J. Pharm. 234:113–119 (2002).
K. Larsen, L. Duedahl-Olesen, S. J. H. Christensen, F. Mathiesen, L. Pedersen, and W. Zimmermann. Purification and characterization of a cyclodextrin glycosyltransferase from Paenibacillus sp. F8. Carbohydr. Res. 310:211–219 (1998).
A. Biwer, G. Antranikian, and E. Heinzle. Enzymatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 59(6):609–617 (2002).
J. Szejtli. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98:1743–1753 (1998).
T. Loftsson, and M. E. Brewster. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85(10):1017–1025 (1996).
V. J. Stella, and R. A. Rajewski. Cyclodextrins: their future in drug formulation and delivery. Pharm. Res. 14(5):556–567 (1997).
T. Higuchi, and K. Connors. Phase solubility diagram. Adv. Anal. Chem. Instrum. 4:117–212 (1965).
T. A. S. Brandao, A. Malheiros, J. D. Magro, V. C. Filho, and R. A. Yunes. Characterization of sesquiterpene polygodial-beta cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 46:77–81 (2003).
K. Miyake, T. Irie, H. Arima, et al. Characterization of itraconazole/2-hydroxypropyl-Beta-cyclodextrin inclusion complex in aqueous propylene glycol solution. Int. J. Pharm. 179(2):237–245 (1999).
H-J. Schneider, F. Hacket, and V. Rudiger. NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98:1755–1785 (1998).
N. Song, and Z. Wang. Synthesis, characterization, and multilayer assemblies of acid and base polyimides. Macromolecules 36:5885–5890 (2003).
T. Loftsson, M. Masson, and M. Brewster. Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91(11):2307–2316 (2002).
K. Uekama, and M. Otagiri. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carr. Syst. 3(1):1–40 (1987).
Y. Sueishi, M. Kasahara, M. Inoue, and K. Matsueda. Effects of substituent and solvent on inclusion complexation of β-cyclodextrins with azobenzene derivatives. J. Incl. Phenom. Macrocycl. Chem. 46(1–2):71–75 (2003).
M. Suzuki, H. Ohmori, M. Kajtar, J. Szejtli, and M. Vikmon. The association of inclusion complexes of cyclodextrins with azo dyes. J. Incl. Phenom. Macrocycl. Chem. 18(3):255–264 (1994).
D. D. Chow, and A. H. Karara. Characterization, dissolution and bioavailability in rats of ibuprofen-beta-cyclodextrin complex system. Int. J. Pharm. 28:95–101 (1986).
E. Iglesias. Inclusion complexation of novocaine by beta-cyclodextrin in aqueous solutions. J. Org. Chem. 71(12):4383–4392 (2006).
J. Liu, L. Qiu, J. Gao, and Y. Jin. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-beta-cyclodextrin. Int. J. Pharm. 312(1–2):137–143 (2006).
M. K. Ghorab, and M. C. Adeyeye. Enhancement of ibuprofen dissolution via wet granulation with beta-cyclodextrin. Pharm. Dev. Technol. 6(3):305–314 (2001).
C. Rodríguez-Tenreiro, C. Alvarez-Lorenzo, A. Concheiro, and J. J. Torres-Labandeira. Characterization of cyclodextrin carbopol interactions by DSC and FTIR. J. Therm. Anal. Calorim. 77:403–411 (2004).
G. H. Hsiue, C. M. Liao, and S. Y. Lin. Effect of drug–polymer interaction on the release characteristics of methacrylic acid copolymer microcapsules containing theophylline. Artif. Organs 22(8):651 (1998).
N. Sarisuta, P. Lawanprasert, S. Puttipipatkhachorn, and K. Srikummoon. The influence of drug–excipient and drug–polymer interactions on butt adhesive strength of ranitidine hydrochloride film-coated tablets. Drug Dev. Ind. Pharm. 32(4):463–471 (2006).
T. Matsui, H. Iwasaki, K. Matsumoto, and Y. Osajima. NMR studies of cyclodextrin inclusion complex with ethyl hexanoate in ethanol solution. Biosci. Biotech. Biochem. 58:1102–1106 (1994).
Acknowledgements
We gratefully acknowledge Drs. Billy Day, Stephen Weber, and Bernard Moncla for providing access to critical instrumentation for use in these studies. We also acknowledge CONRAD for providing UC781 for these studies. This work was supported in part by NIH grant AI051661.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Parniak, M.A., Isaacs, C.E. et al. Characterization of Cyclodextrin Inclusion Complexes of the Anti-HIV Non-Nucleoside Reverse Transcriptase Inhibitor UC781. AAPS J 10, 606–613 (2008). https://doi.org/10.1208/s12248-008-9070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-008-9070-3