Article contents
Dzierżanowskite, CaCu2S2 – a new natural thiocuprate from Jabel Harmun, Judean Desert, Palestine Autonomy, Israel
Published online by Cambridge University Press: 02 January 2018
Abstract
Dzierżanowskite, 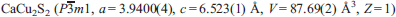 , a thiocuprate, was found in larnite pseudoconglomerate rocks of the Hatrurim Complex at Jabel Harmun, Palestinian Autonomy, Israel. Dzierżanowskite occurs in larnite pebbles, which are embedded in a low-temperature mineral matrix. Associated minerals are larnite, brownmillerite, fluorellestadite, ye'elimite, gehlenite, periclase, ternesite, nabimusaite, vorlanite, vapnikite, fluormayenite, fluorkyuygenite, oldhamite, jasmundite, covellite, chalcocite and pyrrhotite. Electron microprobe analyses yield an average composition of Cu 55.25, Fe 0.13, S 27.46 and Ca 16.99, total 99.83 wt.%. The empirical formula of dzierżanowskite, based on 5 atoms, is Ca0.98Cu2.02Fe0.01S1.99. Dzierżanowskite forms grains up to 15 μm in size or rims on oldhamite and laminar intergrowths with chalcocite and covellite. Dzierżanowskite is dark orange, has a cream streak and a submetallic lustre. In reflected light it is grey, with a cream tint and characteristic yellow-orange internal reflections. The calculated density of dzierżanowskite is 4.391 g cm -3. Three bands at 300, 103 and 86 cm -1 are observed in the Raman spectrum. The strongest lines of the calculated powder diffraction pattern are [d, Å (I) hkl]: 2.358(100) 102, 1.970(93)110, 3.023(78) 011, 6.523(36) 001, 3.412 (28) 100, 1.834(28) 103. Dzierżanowskite was also found in unusual jasmundite rocks, forming small ‘paleofumaroles’ within areas of low-temperature hydrothermal rocks bearing larnite pseudoconglomerates at Jabel Harmun. Dzierżanowskite is a superimposed phase of the high-temperature alteration of pyrometamorphic rocks subjected to by-products (melts/fluids and gases) of pyrometamorphism originating in the deeper levels of combustion.
, a thiocuprate, was found in larnite pseudoconglomerate rocks of the Hatrurim Complex at Jabel Harmun, Palestinian Autonomy, Israel. Dzierżanowskite occurs in larnite pebbles, which are embedded in a low-temperature mineral matrix. Associated minerals are larnite, brownmillerite, fluorellestadite, ye'elimite, gehlenite, periclase, ternesite, nabimusaite, vorlanite, vapnikite, fluormayenite, fluorkyuygenite, oldhamite, jasmundite, covellite, chalcocite and pyrrhotite. Electron microprobe analyses yield an average composition of Cu 55.25, Fe 0.13, S 27.46 and Ca 16.99, total 99.83 wt.%. The empirical formula of dzierżanowskite, based on 5 atoms, is Ca0.98Cu2.02Fe0.01S1.99. Dzierżanowskite forms grains up to 15 μm in size or rims on oldhamite and laminar intergrowths with chalcocite and covellite. Dzierżanowskite is dark orange, has a cream streak and a submetallic lustre. In reflected light it is grey, with a cream tint and characteristic yellow-orange internal reflections. The calculated density of dzierżanowskite is 4.391 g cm -3. Three bands at 300, 103 and 86 cm -1 are observed in the Raman spectrum. The strongest lines of the calculated powder diffraction pattern are [d, Å (I) hkl]: 2.358(100) 102, 1.970(93)110, 3.023(78) 011, 6.523(36) 001, 3.412 (28) 100, 1.834(28) 103. Dzierżanowskite was also found in unusual jasmundite rocks, forming small ‘paleofumaroles’ within areas of low-temperature hydrothermal rocks bearing larnite pseudoconglomerates at Jabel Harmun. Dzierżanowskite is a superimposed phase of the high-temperature alteration of pyrometamorphic rocks subjected to by-products (melts/fluids and gases) of pyrometamorphism originating in the deeper levels of combustion.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2017
References
- 11
- Cited by




