Abstract
Background
Pharmacovigilance of patient support programs (PSPs) has been the subject of debate, legislation, and guidance, and regulatory inspections over the last half-a-dozen years. PSPs often involve direct contact between patients or caregivers and health care professionals and are sponsored by marketing authorization holders (MAHs). PSPs have no scientific hypothesis under study, and are not governed by a protocol. Adverse events and suspected adverse reactions are expected to arise. Management of safety data is governed by a patchwork of incongruous guidelines worldwide, leaving room for varying interpretations.
Methods
A survey of MAHs was conducted to inquire about methods, techniques and scope of pharmacovigilance activities concerning PSPs and similar organized data collection systems. The survey was conducted over a 6-week period by contacting pharmacovigilance operations managers in a broad range of MAHs.
Results
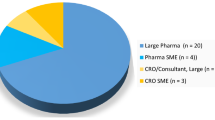
The survey was completed by 18 of 35 MAHs. The vast majority of MAHs (94%) conduct market research. Patient support programs were sponsored or supported by 89% of the respondents, with 67% of MAHs sponsoring or supporting disease management programs or social media resources.
Conclusion
Pharmaceutical industry responses to the challenge of pharmacovigilance of patient support programs are varied. In general there has been a consistent response to the European pharmacovigilance regulations and the accompanying guidance introduced in June 2012. As evidenced by the following survey results, many companies have adopted a risk-based approach first assessing each PSP for probability of AE generation, and then setting up a contract and processes to ensure appropriate collection, collation, and assessment of reports of suspected adverse reactions. At the same time, the survey results indicate that many companies are not as mature in their oversight of PSPs. The authors recommend collaboration within the industry to define and agree to industry standard approaches for oversight of PSPs and the adoption of evidence-based simplification of the current regulatory guidelines concerning safety monitoring and reporting, as the current burden is onerous, and over a period of several years has not yielded any information on medically important new risks. Specifically, the authors recommend the formation of a CIOMS Expert Working Group to spearhead this industry collaboration to find a better path forward.
Similar content being viewed by others
References
European Medicines Agency [Internet]. Good Pharmacovigilance Practices, Module VI, Revision 1, Management and reporting of adverse reactions to medicinal products. EMA/873138/2011. September 2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500172402.pdf. Accessed September 17, 2016.
EMA Workshop on patient support and market research programs [Internet]. http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2013/06/WC500144667.pdf. Accessed September 17, 2016.
Roche [Internet]. Annual Report 2012. http://www.roche.com/gb12e.pdf. Accessed September 17, 2016.
MHRA Good Pharmacovigilance Practice Inspectorate [Internet]. Annual inspection reports 2009-2014. https://www.gov.uk/government/statistics/pharmacovigilance-inspection-metrics-2009-to-present. Accessed September 17, 2016.
European Medicines Agency [Internet]. European Medicines Agency acts on deficiencies in Roche medicines-safety reporting. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2012/06/news_detail_001539.jsp&mid=WC0b01ac058004d5c1. Accessed September 17, 2016.
Sloane R, Osanlou O, Lewis D, Bollegala D, Maskell S, Pirmohamed M. Social media and pharmacovigilance: a review of the opportunities and challenges. Br J Clin Pharmacol 2015;80(4):910–920.
European Parliament and Council [Internet]. Directive 2001/83. http://ec.europa.eu/health/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf. Accessed September 17, 2016.
European Parliament and Council [Internet]. Regulation 726/2044. http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0726&from=EN. Accessed September 17, 2016.
European commission [Internet]. Implementing Regulation 520/2012. http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:159:0005:0025:EN:PDF. Accessed September 17, 2016.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Portnoff, J.M., Lewis, D.J. The Enigma of Pharmacovigilance of Patient Support Programs: A Survey of Marketing Authorization Holders in Europe. Ther Innov Regul Sci 51, 486–493 (2017). https://doi.org/10.1177/2168479017696264
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479017696264