Abstract
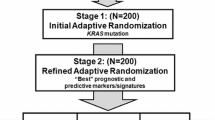
In this paper, the authors describe developments in adaptive design methodology and discuss implementation strategies and operational challenges in early-phase adaptive clinical trials. The BATTLE trial—the first completed biomarker-based Bayesian adaptive randomized study in lung cancer—is presented as a case study to illustrate main ideas and share learnings.
Similar content being viewed by others
References
Ashby D. Bayesian statistics in medicine: a 25 year review. Stat Med. 2006;25(21):3589–3631.
Grieve AP. 25 years of Bayesian methods in the pharmaceutical industry: a personal, statistical bummel. Pharm Stat. 2007;6(4):261–281.
Chevret S. Bayesian adaptive clinical trials: a dream for statisticians only? Stat Med. 2012;31(11–12):1002–1013.
Lee JJ, Chu CT. Bayesian clinical trials in action. Stat Med. 2012;31(25):2955–2972.
Zhou X, Liu S, Kim ES, Herbst RS, Lee JJ. Bayesian adaptive design for targeted therapy development in lung cancer: a step toward personalized medicine. Clin Trials. 2008;5(3):463–467.
Kim ES, Herbs RS, Wistuba II, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1(1):44–53.
Barker AD, Sigman CC, Kelloff GJ, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86(1):97–100.
Gaydos B, Anderson K, Berry D, et al. Good practices for adaptive clinical trials in pharmaceutical product development. Drug Inf J. 2009;43:539–556.
He W, Kuznetsova O, Harmer M, et al. Practical considerations and strategies for executing adaptive clinical trials. Drug Inf J. 2012;46:160–174.
Dragalin V. Adaptive designs: terminology and classification. Drug Inf J. 2006;40:425–435.
US Food and Drug Administration. Guidance for industry 2010: adaptive design clinical trials for drugs and biologics. https://www.fda.gov/downloads/DrugsGuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf.
Hu F, Rosenberger W. The Theory of Response-Adaptive Randomization in Clinical Trials. New York, NY: Wiley; 2006.
Rosenberger W, Lachin J. Randomization in Clinical Trials, Theory, and Practice. New York, NY: Wiley; 2002.
Rosenberger W, Sverdlov O, Hu F. Adaptive randomization for clinical trials. J Biopharm Stat. 2012;22(4):719–736.
Albert JH, Chib S. Bayesian analysis of binary and polychotomous response data. J Am Stat Assoc. 1993;88:669–679.
Berry D, Eick S. Adaptive assignment versus balanced randomization in clinical trials: a decision analysis. Stat Med. 1995;14:231–246.
Thall P, Wathen J. Practical Bayesian adaptive randomization in clinical trials. Eur J Cancer. 2007;43:859–866.
Wathen J, Cook J. Power and Bias in Adaptively Randomized Clinical Trials. Houston, TX: Department of Biostatistics, MD Anderson Cancer Center; 2006.
Berry D, Carlin L, Lee JJ, Muller P. Bayesian Adaptive Methods for Clinical Trials. New York, NY: CRC Press; 2011.
Ghosh B, Sen PK. Handbook of Sequential Analysis. New York, NY: Marcel Dekker Inc; 1991.
Jennison C, Turnbull BW. Group Sequential Methods With Applications to Clinical Trials. New York, NY: CRC Press Inc; 2000.
Proshan MA, Lan KKG, Wittes JT. Statistical Monitoring of Clinical Trials: A Unified Approach. New York, NY: Springer; 2006.
Thall P, Simon R. Practical Bayesian guidelines for phase IIB clinical trials. Biometrics. 1994;50:337–349.
Thall P, Simon R, Estey E. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14:357–379.
Lee J, Liu D. A predictive probability design for phase II cancer clinical trials. Clin Trials. 2008;5:93–106.
Wetherill G. Sequential estimation of quantal response curves. J Royal Stat Soc B. 1963;25:1–48.
Lai TL, Robbins H. Adaptive design in regression and control. Proc Natl Acad Sci U S A. 1978;75:586–587.
O’Quigley J, Pepe M, Fisher L. Continual reassessment method: A practical design for phase 1 clinical trials in cancer. Biometrics.1990;46:33–48.
Li Z, Durham SD, Flournoy N. An adaptive design for maximization of a contingent binary response. In Flournoy N, Rosenberger WF, eds. Adaptive Designs. Beachwood, OH: Institute of Mathematical Statistics; 1995:179–196.
Thall PF, Cook JD. Dose-finding based on efficacy? Toxicity trade-offs. Biometrics. 2004;60(3):684–693.
Azriel D. A note on the robustness of the continual reassessment method. Stat Prob Lett. 2012;82:902–906.
Azriel D, Mandel M, Rinott Y. The treatment versus experimentation dilemma in dose finding studies. J Stat Plan Infer. 2011;141(8):2759–2768.
Bozin A, Zarrop M. Self-tuning extremum optimizer convergence and robustness. ECC. 1991;91:672–677.
Chang HH, Ying Z. Nonlinear sequential designs for logistic item response theory models with applications to computerized adaptive tests. Annals Stat. 2009;37(3):1466–1488.
Ghosh M, Mukhopadhyay N, Sen PK. Sequential Estimation. New York, NY: Wiley; 1997.
Lai TL, Robbins H. Iterated least squares in multiperiod control. Adv Appl Math. 1982;3(1):50–73.
Oron AP, Azriel D, Hoff PD. Dose-finding designs: the role of convergence properties [published online October 27, 2011]. Int J Biostat.
Pronzato L. Adaptive optimization and D-optimum experimental design. Annals Stat. 2000;28(6):1743–1761.
Gooley TA, Martin PJ, Lloyd DF, Pettinger M. Simulation as a design tool for phase I/II clinical trials: an example from bone marrow transplantation. Control Clin Trials. 1994;15:450–460.
Fan SK, Chaloner K. Optimal designs and limiting optimal designs for a trinomial response. J Stat Plan Infer. 2004;126(1):347–360.
Rabie H, Flournoy N. Optimal designs for contingent responses models. In: Di Bucchianico A, Luter H, Wynn HP, eds. mODa 7: Advances in Model-Oriented Design and Analysis. Heidelberg, germany: Physical-Verlag; 2004:133–142.
Dragalin V, Fedorov V. Adaptive designs for dose-finding based on efficacy-toxicity response. J Stat Plan Infer. 2006;136:1800–1823.
Fedorov V, Leonov S. Optimal Design for Nonlinear Response Models. New York, NY: CRC Press Inc; 2013.
Thall PF. Bayesian models and decision algorithms for complex early phase clinical trials. Stat Sci. 2010;25(2):227–244.
Dragalin V, Fedorov V, Wu Y. Two-stage design for dose-finding that accounts for both efficacy and safety. Stat Med. 2008;27:5156–5176.
Fedorov V, Wu Y, Zhang R. Optimal dose-finding designs with correlated continuous and discrete responses. Stat Med. 2012;31:217–234.
Berry D. Statistics: A Bayesian Perspective. London, England: Duxbury Press; 1996.
Berry D. Bayesian clinical trials. Nat Rev Drug Discov. 2006;5:27–36.
Lin Y, Shih W. Statistical properties of the traditional algorithm-based designs for phase I cancer clinical trials. Biostatistics. 2001;2(2):203–215.
Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17:1103–1120.
Chevret S. Statistical Methods for Dose-Finding Experiments. New York, NY: Wiley; 2006.
Cheung YK. Dose Finding by the Continual Reassessment Method. New York, NY: Chapman. & Hall; 2011.
Wang S, O’Neill R, Hung H. Approaches to evaluation of treatment effect in randomized clinical trials with genomic subset. Pharmaceutical Statistics. 2007;6:227–244.
Lipkovich I, Dmitrienko A, Denne J, Enas G. Subgroup identification based on differential effect search (SIDES): a recursive partitioning method for establishing response to treatment in patient subpopulations. Stat Med. 2011;30:2601–2621.
Lipkovich I, Dmitrienko A. Strategies for identifying predictive biomarkers and subgroups with enhanced treatment effect in clinical trials using SIDES. J Biopharm Stat. In press.
Stallard N. A confirmatory seamless phase II/III clinical trial design incorporating short-term endpoint information. Stat Med. 2010;29:959–971.
Jenkins M, Stone A, Jennison C. An adaptive seamless phase II/III design for oncology trials with subpopulation selection using correlated survival endpoints. Pharm Stat. 2011;10:347–356.
Friede T, Parsons N, Stallard N. A conditional error function approach for subgroup selection in adaptive clinical trials. Stat Med. 2012;31(30):4309–4320.
Lara PN, Redman MW, Kelly KM, et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol. 2008;26(3):463–467.
Sequist LV, Muzikansky A, Engelman JA. A new BATTLE in the evolving war on cancer. Cancer Discov. 2011;1(1):14–16.
Rubin EH, Anderson KM, Gause CK. The BATTLE trial: a bold step toward improving the efficiency of biomarker-based drug development. Cancer Discov. 2011:1(1):17–20.
Tam AL, Kim ES, Lee JJ, et al. Feasibility of image-guided transthoracic core-needle biopsy in the BATTLE lung trial. J Thorac Oncol. 2013;8(4):436–442.
Lee JJ, Chen N, Yin G. Worth adapting? Revisiting the usefulness of outcome-adaptive randomization. Clin Cancer Res. 2012;18(17):4498–4507.
Lee JJ, Gu X, Liu S. Bayesian adaptive randomization designs for targeted agent development. Clin Trials. 2010;7(5):584–596.
Printz C. BATTLE to personalize lung cancer treatment: novel clinical trial design and tissue gathering procedures drive biomarker discovery. Cancer. 2010;116(14):3307–3308.
Gold KA, Kim ES, Lee JJ, Wistuba II, Farhangfar CJ, Hong WK. The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res. 2011;4(7):962–972.
Lai TL, Lavori PW, Shih MCI, Sikic BI. Clinical trial designs for testing biomarker-based personalized therapies. Clin Trials. 2012;9(2):141–154.
Rubin EH, Gilliland DG. Drug development and clinical trials: the path to an approved cancer drug. Nat Rev Clin Oncol. 2012;9(4):215–222.
Kelloff GJ, Sigman CC. Cancer biomarkers: selecting the right drug for the right patient. Nat Rev Drug Discov. 2012;11(3):201–214.
Berry DA, Herbst RS, Rubin EH. Reports from the 2010 clinical and translational cancer research think tank meeting: design strategies for personalized therapy trials. Clin Cancer Res. 2012;18(3):638–644.
Allison M. Reinventing clinical trials. Nat Biotechnol. 2012;30(1):41–49.
US Food and Drug Administration. Innovation/stagnation: challenge and opportunity on the critical path to new medical products. https://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/CriticalPathOpportunitiesReports/ucm077262.htm. Published March 2004.
Bornkamp B, Bretz F, Dmitrienko A, et al. Innovative approaches for designing and analyzing adaptive dose-ranging trials. J Biopharm Stat. 2007;17:965–995.
Pinheiro J, Sax R, Antonijevic Z, et al. Adaptive and model-based dose ranging trials: quantitative evaluation and recommendations. Stat Biopharm Res. 2010;2(4):435–454.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marchenko, O., Fedorov, V., Lee, J.J. et al. Adaptive Clinical Trials. Ther Innov Regul Sci 48, 20–30 (2014). https://doi.org/10.1177/2168479013513889
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/2168479013513889