Abstract
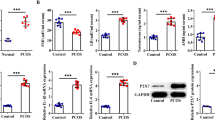
Kisspeptins are a family of neuropeptides that are essential for fertility. Recent experimental data suggest a putative role of kisspeptin signaling in the direct control of ovarian function. To explore the expression of KISS1 and KISS1 receptor (KISS1R) in human granulosa lutein cells and the potential role of KISS1/KISS1R system in the pathogenesis of polycystic ovary syndrome (PCOS), we measured the concentration of KISS1 in follicular fluid, the expression of KISS1 and KISS1R in granulosa lutein cells, and the circulating hormones. The expression levels of KISS1 and KISS1R were significantly upregulated in human granulosa lutein cells obtained from women with PCOS. The expression levels of KISS1 in human granulosa lutein cells highly correlated with those of KISS1R in non-PCOS patients, but not in patients with PCOS, most likely due to the divergent expression patterns in women with PCOS. Additionally, the expression levels of KISS1 highly correlated with the serum levels of anti-Mullerian hormone (AMH). The expression levels of KISS1 and KISS1R, as well as the follicular fluid levels of KISS1, were not significantly different between the pregnant and nonpregnant patients in both PCOS and non-PCOS groups. In conclusion, the increased expression of KISS1 and KISS1R in human granulosa lutein cells may contribute to the pathogenesis of PCOS. The expression levels of KISS1 highly correlated with the serum levels of AMH. The KISS1 and KISS1R system in the ovary may not have a remarkable role in predicting the in vitro fertilization (IVF) outcome.
Similar content being viewed by others
References
Roseweir AK, Millar RP. The role of kisspeptin in the control of gonadotrophin secretion. Hum Reprod Update. 2009;15(2): 203–212.
Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316.
Skorupskaite K, George JT, Anderson RA. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum Reprod Update. 2014;20(4):485–500.
Garcia-Ortega J, Pinto FM, Fernandez-Sanchez M, et al. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod. 2014;29(12):2736–2746.
Peng J, Tang M, Zhang BP, et al. Kisspeptin stimulates progesterone secretion via the Erk1/2 mitogen-activated protein kinase signaling pathway in rat luteal cells. Fertil steril. 2013;99(5): 1436–1443.e1431.
Hussain MA, Song WJ, Wolfe A. There is kisspeptin - and then there is kisspeptin. Trends Endocrinol Metab. 2015;26(10): 564–572.
Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology. 2015;156(4):1218–1227.
Garcia-Ortega J, Pinto FM, Prados N, et al. Expression of tachy-kinins and tachykinin receptors and interaction with kisspeptin in human granulosa and cumulus cells. Biol Reprod. 2016;94(6): 124.
Merhi Z, Thornton K, Bonney E, Cipolla MJ, Charron MJ, Buyuk E. Ovarian kisspeptin expression is related to age and to monocyte chemoattractant protein-1. J Assist Reprod Genet. 2016;33(4): 535–543.
Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod. 2015;93(1):15.
Cejudo Roman A, Pinto FM, Dorta I, et al. Analysis ofthe expression of neurokinin B, kisspeptin, and their cognate receptors NK3 R and KISS1 R in the human female genital tract. Fertil steril. 2012;97(5):1213–1219.
Hu K-L, Zhao H, Chang H-M, Yu Y, Qiao J. Kisspeptin/kisspep- tin receptor system in the ovary. Front Endocrinol. 2018;8:365.
Saadeldin IM, Koo OJ, Kang JT, et al. Paradoxical effects of kisspeptin: it enhances oocyte in vitro maturation but has an adverse impact on hatched blastocysts during in vitro culture. Reprod Fertil Dev. 2012;24(5):656–668.
Gaytan F, Garcia-Galiano D, Dorfman MD, et al. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology. 2014;155(8):3088–3097.
Castellano JM, Gaytan M, Roa J, et al. Expression ofKiSS-1 in rat ovary: putative local regulator of ovulation?. Endocrinology. 2006;147(10):4852–4862.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fer-til Steril. 2004;81(1):19–25.
Richards JS, Ascoli M. Endocrine, paracrine, and autocrine signaling pathways that regulate ovulation. Trends Endocrinol Metab. 2018;29(5):313–325.
Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, et al. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: a comparative study. Hum Reprod. 2012;27(6):1781–1789.
Merhi Z, Buyuk E, Berger DS, et al. Leptin suppresses anti-Mullerian hormone gene expression through the JAK2/STAT3 pathway in luteinized granulosa cells of women undergoing IVF. Hum Reprod. 2013;28(6):1661–1669.
Jeon YE, Lee KE, Jung JA, et al. Kisspeptin, leptin, and retinol-binding protein 4 in women with polycystic ovary syndrome. Gynecol Obstet Inves. 2013;75(4):268–274.
Chen X, Mo Y, Li L, Chen Y, Li Y, Yang D. Increased plasma metastin levels in adolescent women with polycystic ovary syndrome. Eur J Obstet Gyn R B. 2010;149(1):72–76.
Yilmaz SA, Kerimoglu OS, Pekin AT, et al. Metastin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gyn R B. 2014;180: 56–60.
Mondal M, Baruah KK, Prakash BS. Determination of plasma kisspeptin concentrations during reproductive cycle and different phases of pregnancy in crossbred cows using bovine specific enzyme immunoassay. Gen Comp Endocrinol. 2015;224: 168–175.
Mondal M, Karunakaran M, Baruah KK. Development and validation of a sensitive enzymeimmunoassay for determination of plasma metastin in mithun (Bos frontalis). J Immunoass Immu- noch. 2016;37(2):201–216.
Li R, Zhang Q, Yang D, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. 2013;28(9):2562–2569.
Kolodziejski PA, Pruszynska-Oszmalek E, Korek E, et al. Serum levels of spexin and kisspeptin negatively correlate with obesity and insulin resistance in women. Physiol Res. 2018;67(1):45–56.
Panidis D, Rousso D, Koliakos G, et al. Plasma metastinlevels are negatively correlated with insulin resistance and free androgens in women with polycystic ovary syndrome. Fertil Steril. 2006;85(6): 1778–1783.
Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2016;228(3):161–170.
Pineda R, Garcia-Galiano D, Roseweir A, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722–730.
Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. New Engl J Med. 2003;349(17):1614–1627.
Acknowledgments
The authors appreciate the help in statistical analysis from Professor Zhao in Peking University Third Hospital.
Author information
Authors and Affiliations
Corresponding author
Supplemental Material
Supplemental Material
Supplemental material for this article is available online.
Rights and permissions
About this article
Cite this article
Hu, KL., Zhao, H., Min, Z. et al. Increased Expression of KISSI and KISSI Receptor in Human Granulosa Lutein Cells—Potential Pathogenesis of Polycystic Ovary Syndrome. Reprod. Sci. 26, 1429–1438 (2019). https://doi.org/10.1177/1933719118818899
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719118818899