Abstract
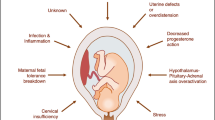
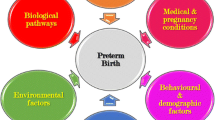
Despite decades of investigations and accumulated scientific knowledge, preterm birth (PTB) remains a significant burden worldwide. Several mechanisms have been proposed to explain this condition, and a number of risk factors from infectious to behavioral and genetic/epigenetic factors influence this outcome. The heritability of PTB is estimated to be 17% to 36%, which demonstrates that genetic predisposition plays a key role in PTB. Structural DNA modifications without changes in the DNA sequence and post-transcriptional regulation also have an impact on gene expression and thus influence pregnancy outcomes. There is a complex interplay between environmental factors and the individual’s genetics and epigenetics that may culminate in PTB, but the complete regulatory pathways and networks involved in this context are still unclear. Here, we outline what is known so far about the genetic and epigenetic factors involved in preterm delivery, including polymorphisms, DNA methylation, and microRNAs, and suggest fields for research.
Similar content being viewed by others
References
Giarratano G. Genetic influences on preterm birth. MCN Am J Matern Child Nurs. 2006;31(3):169–175.
Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lack-ritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573.
Behrman RE, Butler AS, eds. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: The National Academies Collection: Reports funded by National Institutes of Health; 2007.
Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38.
Arpino C, D’Argenzio L, Ticconi C, et al. Brain damage in preterm infants: etiological pathways. Ann 1st Super Sanita. 2005;41(2):229–237.
Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765.
Ramos B de A, Kanninen TT, Sisti G, Witkin SS. Microorganisms in the female genital tract during pregnancy: tolerance versus pathogenesis. Am JReprod Immunol. 2015;73(5):383–389.
Patni S, Flynn P, Wynen LP, et al. An introduction to Toll-like receptors and their possible role in the initiation of labour. BJOG. 2007;14(11):1326–1334.
Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79(1):50–57.
Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(suppl 3): 17–42.
Goldenberg RL, Culhane JF, lams JD, Romero R. Epidemiology and causes of preterm birth. lancet. 2008;371(9606):75–84.
Ramos BR, Mendes ND, Tanikawa AA, et al. Ancestry informative markers and selected single nucleotide polymorphisms in immunoregulatory genes on preterm labor and preterm premature rupture of membranes: a case control study. BMC Pregnancy Childbirth. 2016;16:30.
Karody VR, Le M, Nelson S, et al. A TIR domain receptor-associated protein (TIRAP) variant SNP (rs8177374) confers protection against premature birth. JPerinatol. 2013;33(5):341–346.
Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol. 2000; 182(2):465–472.
Menon R, Boldogh I, Urrabaz-Garza R, et al. Senescence of primary amniotic cells via oxidative DNA damage. PIoS One. 2013;8(12): e83416.
Ferrero DM, Larson J, Jacobsson B, et al. Cross-Country Individual Participant Analysis of 4.1 Million Singleton Births in 5 Countries with Very High Human Development Index Confirms Known Associations but Provides No Biologic Explanation for 2/3 of All Preterm Births. PIoS One. 2016;11(9): e0162506.
Boyd HA, Poulsen G, Wohlfahrt J, et al. Maternal contributions to preterm delivery. Am J Epidemiol. 2009;170(11):1358–1364.
Bhattacharya S, Raja EA, Mirazo ER, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol. 2010;115(6):1125–1133.
Treloar SA, Macones GA, Mitchell LE, Martin NG. Genetic influences on premature parturition in an Australian twin sample. Twin Res. 2000;3(2):80–82.
Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG. 2000;107(3):375–381.
Anum EA, Springel EH, Shriver MD, Strauss JF III. Genetic contributions to disparities in preterm birth. Pediatr Res. 2009;65(1):1–9.
Vinikoor LC, Kaufman JS, MacLehose RF, Laraia BA. Effects of racial density and income incongruity on pregnancy outcomes in less segregated communities. Soc Sci Med. 2008;66(2):255–259.
Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25(1):21–39.
Hoist D, Gamier Y. Preterm birth and inflammation- The role of genetic polymorphisms. Eur J Obstet Gynecol Reprod Biol. 2008; 141(1):3–9.
Wilcox AJ, Skaerven R, Lie RT. Familial patterns of preterm delivery: Maternal and fetal contributions. Am J Epidemiol. 2008;167(4):474–479.
Svensson AC, Sandin S, Cnattingius S, et al. Maternal effects for preterm birth: A genetic epidemiologic study of 630,000 families. Am J Epidemiol. 2009;170(11):1365–1372.
Wang H, Ogawa M, Wood JR, et al. Genetic and epigenetic mechanisms combine to control MMP1 expression and its association with preterm premature rupture of membranes. Hum Mol Genet. 2008;17(8):1087–1096.
Kim J, Pitlick MM, Christine PJ, et al. Genome-wide analysis of DNA methylation in human amnion. Scientific World Journal. 2013;2013:67815.
Yilmaz Y, Verdi H, Taneri A, et al. Maternal-fetal proinflammatory cytokine gene polymorphism and preterm birth. DNA Cell Biol. 2012;31(1):92–97.
Schmid M, Haslinger P, Stary S, Leipold H, Egarter C, Grimm C. Interleukin-1 beta gene polymorphisms and preterm birth. Eur J Obstet Gynecol Reprod Biol. 2012;165(1):33–36.
Jones NM, Holzman C, Tian Y, et al. Innate immune system gene polymorphisms in maternal and child genotype and risk of preterm delivery. J Matern Fetal Neonatal Med. 2012;25(3):240–247.
Jafarzadeh L, Danesh A, Sadeghi M, Heybati F, Hashemzadeh M. Analysis of Relationship between Tumor Necrosis Factor Alpha Gene (G308A Polymorphism) with Preterm Labor. Int J Prev Med. 2013;4(8):896–901.
Moura E, Mattar R, de Souza E, Torloni MR, Goncalves-Primo A, Daher S. Inflammatory cytokine gene polymorphisms and spontaneous preterm birth. J Reprod Immunol. 2009;80(1-2):115–121.
Varner MW, Esplin MS. Current understanding of genetic factors in preterm birth. BJOG. 2005;112(suppl 1):28–31.
Liang M, Wang X, Li J, et al. Association of combined maternal-fetal TNF-alpha gene G308A genotypes with preterm delivery: a gene-gene interaction study. J Biomed Biotechnol. 2010;2010: 396184.
El-Bastawissi AY, Williams MA, Riley DE, Hitti J, Krieger JN. Amniotic fluid interleukin-6 and preterm delivery: a review. Obstet Gynecol. 2000;95(6 pt 2): 1056–1064.
Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82(5):423–431.
Kalinka J, Bitner A. Selected cytokine gene polymorphisms and the risk of preterm delivery in the population of Polish women. GinekolPol. 2009;80(2):111–117.
Velez DR, Fortunate SJ, Williams SM, Menon R. Interleukin-6 (IL-6) and receptor (IL6-R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum Mol Genet. 2008;17(11):1619–1630.
Speer EM, Gentile DA, Zeevi A, Pillage G, Huo D, Skoner DP. Role of single nucleotide polymorphisms of cytokine genes in spontaneous preterm delivery. Hum Immunol. 2006;67(11):915–923.
Abrahams VM, Aldo PB, Murphy SP, et al. TLR6 modulates first trimester trophoblast responses to peptidoglycan. J Immunol. 2008;180(9):6035–6043.
Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl AcadSci USA. 2000;97(25):13766–13771.
Koga K, Mor G. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy disorders. Am J Reprod Immunol. 2010;63(6):587–600.
Sutherland AM, Walley KR, Russell JA. Polymorphisms in CD14, mannose-binding lectin, and toll-likereceptor-2 are associated with increased prevalence of infection in critically ill adults. Crit Care Med. 2005;33(3):638–644.
Bitner A, Sobala W, Kalinka J. Association between maternal and fetal TLR4 (896A>G, 1196C>T) gene polymorphisms and the risk of preterm birth in the Polish population. Am J Reprod Immunol. 2013;69(3):272–280.
Arbour NC, Lorenz E, Schutte BC, et al. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet. 2000;25(2):187–191.
Manuck TA, Major HD, Varner MW, Chettier R, Nelson L, Esplin MS. Progesterone receptor genotype, family history, and spontaneous preterm birth. Obstet Gynecol. 2010;115(4):765–770.
Mann PC, Cooper ME, Ryckman KK, et al. Polymorphisms in the fetal progesterone receptor and a calcium-activated potassium channel isoform are associated with preterm birth in an Argentinian population. JPerinatol. 2013;33(5):336–340.
Diaz-Cueto L, Dominguez-Lopez P, Cantillo-Cabarcas J, Perez-Figueroa G, Arechavaleta-Velasco M, Arechavaleta-Velasco F. Progesterone receptor gene polymorphisms are not associated with preterm birth in a Hispanic population. Int J Gynaecol Obstet. 2008;103(2):153–157.
Kuessel L, Grimm C, Knöfler M, et al. Common oxytocin receptor gene polymorphisms and the risk for preterm birth. Dis Markers. 2013;34(1):51–56.
Haataja R, Karjalainen MK, Luukkonen A, et al. Mapping a new spontaneous preterm birth susceptibility gene, IGF1 R, using linkage, haplotype sharing, and association analysis. PIoS Genet. 2011;7(2):e1001293.
Karjalainen MK, Huusko JM, Ulvila J, et al. A potential novel spontaneous preterm birth gene, AR, identified by linkage and association analysis of X chromosomal markers. PIoS One. 2012;7(12): e51378.
Sheikh IA, Ahmad E, Jamal MS, et al. Spontaneous preterm birth and single nucleotide gene polymorphisms: a recent update. BMC Genomics. 2016;17(suppl 9):759.
Alleman BW, Myking S, Ryckman KK, et al. No observed association for mitochondrial SNPs with preterm delivery and related outcomes. Pediatr Res. 2012;72(5):539–544.
Ramos BR, D’Elia MP, Amador MA, et al. Neither self-reported ethnicity nor declared family origin are reliable indicators of genomic ancestry. Genetica. 2016;144(3):259–265.
Parets SE, Conneely KN, Kilaru V, Menon R, Smith AK. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics. 2015;10(9):784–792.
Parets SE, Conneely KN, Kilaru V, et al. Fetal DNA Methylation Associates with Early Spontaneous Preterm Birth and Gestational Age. PIoS One. 2013;8(6): e67489.
McKay JA, Wong YK, Relton CL, Ford D, Mathers JC. Maternal folate supply and sex influence gene-specific DNA methylation in the fetal gut. Mol Nutr Food Res. 2011;55(11):1717–1723.
Maloney CA, Hay SM, Rees WD. Folate deficiency during pregnancy impacts on methyl metabolism without affecting global DNA methylation in the rat fetus. BrJNutr. 2007;97(6):1090–1098.
Timmermans S, Jaddoe VW, Hofman A, Steegers-Theunissen RP, Steegers EA. Periconception folic acid supplementation, fetal growth and the risks of lowbirth weight and preterm birth: the Generation R Study. BrJNutr. 2009;102(5):777–785.
Chiaffarino F, Ascone GB, Bortolus R, et al. Effects of folic acid supplementation on pregnancy outcomes: a review of randomized clinical trials [in Italian]. Minerva Ginecol. 2010;62(4):293–301.
Porter TR, Kent ST, Su W, Beck HM, Gohlke JM. Spatio temporal association between birth outcomes and coke production and steel making facilities in Alabama, USA: a cross-sectional study. Environ Health. 2014;13:85.
Ferguson KK, McElrath TF, Chen YH, Mukherjee B, Meeker JD. Urinary phthalate metabolites and biomarkers of oxidative stress in pregnant women: a repeated measures analysis. Environ Health Perspect. 2015;123(3):210–216.
Li L, Zhang T, Qin XS, et al. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014;41(3):1227–1235.
Burris HH, Baccarelli AA, Wright RO, Wright RJ. Epigenetics: linking social and environmental exposures to preterm birth. Pediatr Res. 2016;79(1-2):136–140.
Sundrani DP, ChavanGautam PM, Mehendale SS, Joshi SR. Altered metabolism of maternal micronutrients and omega 3 fatty acids epigenetically regulate matrix metalloproteinases in preterm pregnancy: a novel hypothesis. Med Hypotheses. 2011;77(5):878–883.
Bartel DP, Micro RNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
Da Sacco L, Masotti A. Recent insights and novel bioinformatics tools to understand the role of microRNAs binding to 5’ untranslated region. Int JMol Sci. 2012;14(1):480–495.
Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455(7209):64–71.
Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008;9(11):831–842.
Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. ReprodSci. 2011;18(1):46–56.
Montenegro D, Romero R, Kim SS, et al. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J Pathol. 2009;217(1):113–121.
Renthal NE, Chen CC, Williams KC, et al. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA. 2010;107(48):20828–20833.
Smith R, Paul J, Maiti K, Tolosa J, Madsen G. Recent advances in understanding the endocrinology of human birth. Trends Endocrinol Metab. 2012;23(10):516–523.
Elovitz MA, Anton L, Bastek J, Brown AG. Can micro RNA profiling in maternal blood identify women at risk for preterm birth? Am J Obstet Gynecol. 2015;212(6):782.e1–e5.
Sanders AP, Gennings C, Svensson K, et al. Bacterial and cytokine mixtures predict the length of gestation and are associated with miRNA expression in the cervix. Epigenomics. 2017;9(1):33–45.
Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci USA. 2012;109(19):7529–7534.
Enquobahrie DA, Hensley M, Qiu C, et al. Candidate gene and microRNA expression in fetal membranes and preterm delivery risk. ReprodSci. 2016;23(6):731–737.
Haneklaus M, Gerlic M, O’Neill LA, Masters SL. miR-223: infection, inflammation and cancer. J Intern Med. 2013;274(3):215–226.
Garg M, Potter JA, Abrahams VM. Identification of microRNAs that regulate TLR2-mediated trophoblast apoptosis and inhibition of IL-6 mRNA. PIoS One. 2013;8(10):e77249.
Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260.
Rudov A, Balduini W, Carloni S, Perrone S, Buonocore G, Albertini MC. Involvement of miRNAs in placental alterations mediated by oxidative stress. Oxid Med Cell longev. 2014; 2014:103068.
Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196(3): 261.e1–e6.
Devlin C, Greco S, Martelli F, Ivan M. MiR-210: more than a silent player in hypoxia. IUBMB life. 2011;63(2):94–100.
Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol. 2014;5:567.
de Andrade Ramos BR, Witkin SS. The influence of oxidative stress and autophagy cross regulation on pregnancy outcome. Cell Stress Chaperones. 2016;21(5):755–762.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Andrade Ramos, B.R., da Silva, M.G. The Burden of Genetic and Epigenetic Traits in Prematurity. Reprod. Sci. 25, 471–479 (2018). https://doi.org/10.1177/1933719117718270
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719117718270