Abstract
Introduction
Apelin is a potent inotropic agent and causes endothelium-mediated vasodilation. Its cardiovascular profile suggests a role in the regulation of gestational hemodynamics.
Methods
We longitudinally assessed maternal serum apelin levels and hemodynamics (cardiac output and total peripheral resistance) between 20 and 34 weeks gestation in 18 women at high risk of placental dysfunction. Placental apelin staining was assessed by immunohistochemistry in placentas from uncomplicated pregnancies (n = 6), preterm deliveries (n = 6), preeclampsia (PET, n = 8), and isolated intrauterine growth restriction (IUGR, n = 8). Placental apelin gene expression was assessed by quantitative polymerase chain reaction.
Results
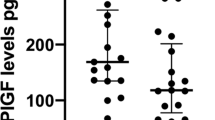
In the high-risk cohort, 4 fetuses developed isolated IUGR and 6 women developed PET. We obtained a median of 5 (range 2-9) hemodynamic and apelin measurements per woman. Apelin levels throughout gestation were best fitted by a quadratic curve. Apelin levels between 20 and 26 weeks gestation correlated with total peripheral resistance (r = .57, P = .01) and showed a trend toward an inverse correlation with stroke volume (r = −.42, P = .08). Apelin serum levels were 30% lower in pregnancies complicated by IUGR than in uncomplicated pregnancies or in women with preeclampsia (P = .009). Placental apelin gene expression was similar in IUGR, PET, preterm, and term normal placentas. Apelin staining was seen both in syncytiotrophoblast and stroma of the placental villi. In IUGR placentas, apelin staining was strongly decreased in both compartments compared to normals. Preeclamptic placentas showed an intermediate staining.
Conclusions
Apelin levels mirror the cardiovascular changes seen in pregnancy. Serum and placental apelin levels are decreased in IUGR.
Similar content being viewed by others
References
Savu O, Jurcut R, Giusca S, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging. 2012;5(3):289–297.
Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension. 2006;47(2):203–208.
Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52(5):873–880.
Schrier RW, Niederberger M. Paradoxes of body fluid volume regulation in health and disease. A unifying hypothesis. West J Med. 1994;161(4):393–408.
Barnes G, Japp AG, Newby DE. Translational promise of the apelin—APJ system. Heart 2010;96(13):1011–1016.
Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008;74(4):486–494.
Habata Y, Fujii R, Hosoya M, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452(1):25–35.
Hosoya M, Kawamata Y, Fukusumi S, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J Biol Chem. 2000;275(28):21061–21067.
Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev. 2010;62(3):331–342.
Vickers C, Hales P, Kaushik V, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277(17):14838–14843.
Van Mieghem T, van Bree R, Van Herck E, Pijnenborg R, Deprest J, Verhaeghe J. Maternal apelin physiology during rat pregnancy: the role of the placenta. Placenta 2010;31(8):725–730.
Proctor LK, Toal M, Keating S, et al. Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2009;34(3):274–282.
Spencer K, Yu CK, Savvidou M, et al. Prediction of preeclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein-A, free beta-human chorionic gonadotropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27(6):658–663.
Albaiges G, Missfelder-Lobos H, Parra M, Lees C, Cooper D, Nicolaides KH. Comparison of color Doppler uterine artery indices in a population at high risk for adverse outcome at 24 weeks’ gestation. Ultrasound Obstet Gynecol. 2003;21(2):170–173.
Stott D, Bolten M, Salman M, Paraschiv D, Clark K, Kametas NA. Maternal demographics and hemodynamics for the prediction of fetal growth restriction at booking, in pregnancies at high risk for placental insufficiency [published online November 24, 2015]. Acta Obstet Gynecol Scand. 2015. doi: 10.1111/aogs.12823.
Drewlo S, Levytska K, Kingdom J. Revisiting the housekeeping genes of human placental development and insufficiency syndromes. Placenta. 2012;33(11):952–954.
Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an opensource platform for biological-image analysis. Nat Methods. 2012;9(7):676–682.
Doherty A, Carvalho JC, Drewlo S, et al. Altered hemodynamics and hyperuricemia accompany an elevated sFlt-1/PlGF ratio before the onset of early severe preeclampsia. J Obstet Gynaecol Can. 2014;36(8):692–700.
Bortoff KD, Qiu C, Runyon S, Williams MA, Maitra R. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy. 2012;31(4):398–404.
Simsek Y, Celik O, Yilmaz E, et al. Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J Matern Fetal Neonatal Med. 2012;25(9):1705–1708.
Kucur M, Tuten A, Oncul M, et al. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy. 2014;33(4):467–475.
Yamaleyeva LM, Chappell MC, Brosnihan KB, et al. Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2015;309(10):E852–E860.
Inuzuka H, Nishizawa H, Inagaki A, et al. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy. 2013;32(4):410–421.
Cobellis L, De Falco M, Mastrogiacomo A, et al. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol. 2007;22(1):1–8.
Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Maternal cardiovascular impairment in pregnancies complicated by severe fetal growth restriction. Hypertension. 2012;60(2):437–443.
Ashley EA, Powers J, Chen M, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc Res. 2005;65(1):73–82.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Van Mieghem, T., Doherty, A., Baczyk, D. et al. Apelin in Normal Pregnancy and Pregnancies Complicated by Placental Insufficiency. Reprod. Sci. 23, 1037–1043 (2016). https://doi.org/10.1177/1933719116630422
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116630422