Abstract
Aim
Although previous studies found that I -time acute stress applied during follicle maturation impaired oocyte competence, it is unknown whether repeated chronic stress, which is known to cause animal behavioral adaptation, would damage oocytes when applied during follicle growth.
Methods and Results
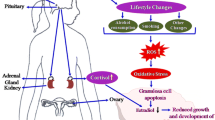
In this study, female mice were exposed to repeated restraint stress (RRS) or unpredictable stress (UPS) for different days before equine chorionic gonadotropin injection to initiate oocyte prematuration development and to observe effects of different Stressors on oocytes in the growing follicles. The results showed that although oocyte pre- and postimplantation development was unaffected when mice were exposed to RRS or UPS once a day for 4 days, development was impaired when mice were exposed to RRS for 8 or more days or to UPS twice a day for 4 days (4 x 2). The 4 x 2 UPS caused more oxidative stress in oocytes and severer apoptosis in antral follicles than did the 4-day RRS. The RRS mice were stressed consistently from days I to 23 of restraint, and the stress that a mouse had 4x2 UPS was severer than that from 4-day RRS.
Conclusion
The results suggest that (I) the degree that a stress damages oocytes is the product of duration x severity of the stress; (2) RRS impaired oocyte developmental potential through cumulative effects on growing follicles; and (3) preantral follicles were not as sensitive to stress as antral follicles were.
Similar content being viewed by others
References
Copper RL, Goldenberg RL, Das A, et al.. The preterm prediction study:maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. Am J Obstet Gynecol. 1996;175(5):1286–1292.
Csemiczky G, Landgren BM, Collins A. The influence of stress and State anxiety on the outcome of IVF-treatment:psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet Gynecol Scand. 2000;79(2):113–118.
Kee BS, Jung BJ, Lee SH. A study on psychological strain in IVF patients. J Assist Reprod Geriet. 2000;17(8):445–448.
Klonoff-Cohen H, Chu E, Natarajan L, Sieber W. A prospective study of stress among women undergoing in vitro fertilization or gamete intrafallopian Transfer. Fertil Steril. 2001;76(4):675–687.
Schröder AK, Katalinic A, Diedrich K, Ludwig M. Cumulative pregnancy rates and drop-out rates in a German IVF programme:4102 cycles in 2130 patients. Reprod Biomed Online. 2004;8(5):600–606.
Neggers Y, Goldenberg R, Cliver S, Hauth J. The relationship between psychosocial profile, health practices, and pregnancy outcomes. Acta Obstet Gynecol Scand. 2006;85(3):277–285.
Tsuma VT, Einarsson S, Madej A, Kindahl H, Lundeheim N, Rojkittikhun T. Endocrine changes during group housing of primipar-ous sows in early pregnancy. Acta Vet Scand. 1996;37(4):481–489.
Peltoniemi OA, Love RJ, Heinonen M, Tuovinen V, Saloniemi H. Seasonal and management effects on fertility of the sow:a descriptive study. Anim Reprod Sci. 1999;55(1):47–61.
Smith J, Ferguson D, Jauregui G, et al.. Shortterm maternal psychological stress in the post-conception period in ewes affects fetal growth and gestation length. Reproduction. 2008;136(2):259–265.
Pare WP, Glavin GB. Restraint stress in biomedical research:a review. Neurosci Biobehav Rev. 1986;10(3):339–370.
Glavin GB, Pare WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research:an update. Neurosci Biobehav Rev. 1994;18(2):223–249.
Wiebold JL, Stanfield PH, Becker WC, Hillers JK. The effect of restraint stress in early pregnancy in mice. J Reprod Fertil. 1986; 78(1):185–192.
Sugino N, Nakamura Y, Okuno N, et al.. Effects of restraint stress on luteal function in rats during mid-pregnancy. J Reprod Fertil. 1994;101(1):23–26.
Mairesse J, Lesage J, Breton C, et al.. Maternal stress alters endocrine funetion of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292(6):E1526–E1533.
Zhang SY, Wang JZ, Li JJ, et al.. Maternal restraint stress diminishes the developmental potential of oocytes. Biol Reprod. 2011;84(4):672–681.
Zhou P, Lian HY, Cui W, et al.. Maternal-restraint stress increases ooeyte aneuploidy by impairing metaphase I spindle assembly and reducing spindle assembly Checkpoint proteins in mice. Biol Reprod. 2012;86(3):83.
Wu LM, Hu MH, Tong XH, et al.. Chronic unpredictable stress decreases expression of brain-derived neurotrophic factor (BDNF) in mouse ovaries:relationship to oocytes developmental potential. PLoS One. 2012;7(12):e52331.
Haleem DJ, Parveen T. Brain regional Serotonin synthesis following adaptation to repeated restraint. Neuroreport. 1994;5(14):1785–1788.
Haleem DJ. Adaptation to repeated restraint stress in rats:failure of ethanoltreated rats to adapt in the stress schedule. Alcohol Alcohol. 1996;31(5):471–477.
Roth Z. Heat stress, the follicle, and its enclosed oocyte:mechanisms and potential strategies to improve fertility in dairy cows. Reprod Dornest Anim. 2008;43(suppl 2):238–244.
Roth Z, Meidan R, Braw-Tal R, Wolfenson D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J Reprod Fertil. 2000;120(1):83–90.
Wu XF, Yuan HJ, Li H, et al.. Restraint stress on female mice diminishes the developmental potential of ooeytes:roles of chromatin configuration and histone modification in germinal vesicle stage ooeytes. Biol Reprod. 2015;92(1):13.
An Y, Sun Z, Li L, Zhang Y, Ji H. Relationship between psychological stress and reproductive outcome in women undergoing in vitro fertilization treatment:psychological and neurohormonal assessment. J Assist Reprod Genet. 2013;30(1):35–41.
Liu YX, Cheng YN, Miao YL, et al.. Psychological stress on female mice diminishes the developmental potential of ooeytes:a study using the predatory stress model. PLoS One. 2012;7(10):e48083.
Liang B, Wei DL, Cheng YN, et al.. Restraint stress impairs ooeyte developmental potential in mice:role of CRH-induced apoptosis of ovarian cells. Biol Reprod. 2013;89(3):64.
Scaramuzzi RJ, Baird DT, Campbell BK, et al.. Regulation of folliculogenesis and the determination of Ovulation rate in rumi-nants. Reprod Fertil Dev. 2011;23(3):444–467.
Monniaux D, Clement F, Dalbies-Tran R, et al.. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles:what is the link? Biol Reprod. 2014;90(4):85.
Huang JH, Liu YY, Chen SJ, et al.. Effect of chronic fatigue on the changes of serum corticosterone. Chin J Trop Med. 2010;10(5):498–501.
Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor Systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–2204.
Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103.
Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18(3):195–200.
Pariante CM. The glucocortieoid reeeptor:part of the Solution or part of the problem? J Psychopharmacol. 2006;20(4 suppl):79–84.
Lian HY, Gao Y, Jiao GZ, et al.. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse ooeytes. Reproduction. 2013; 146(6):559–568.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, Y., Chen, F., Kong, QQ. et al. Stresses on Female Mice Impair Oocyte Developmental Potential:Effects of Stress Severity and Duration on Oocytes at the Growing Follicle Stage. Reprod. Sci. 23, 1148–1157 (2016). https://doi.org/10.1177/1933719116630416
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719116630416