Abstract
Objective
Preeclampsia has been associated with elevated proinflammatory markers, increased sympathetic activity, and decreased plasma volume (PV). We hypothesized that these associations would be identified in women prior to a first pregnancy.
Methods
We studied 76 healthy nulligravid participants measuring the proinflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α). Plasma volume was measured in supine position and corrected for body mass index (BMI). We examined supine plasma levels of epinephrine and norepinephrine and blood pressure response to Valsalva maneuver to quantify sympathetic activation. We then examined the association of PV and sympathetic activity with proinflammatory cytokines with P < .05 accepted for significance.
Results
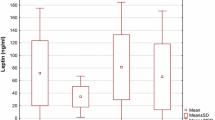
CRP was significantly increased in participants with lowest PV/BMI quartile when compared to middle 2 quartiles and highest quartile (analysis of variance [ANOVA], P = .037). We found no significant association of PV/BMI with either IL-6 or TNF-a. Both plasma epinephrine concentration (r = .29, P = .02) and the late phase II (phase II_L) blood pressure response to Valsalva maneuver (r = .44, P < .0001) were associated with serum IL-6 concentrations.
Conclusions
Low PV is associated with increased CRP levels and increased sympathetic tone is linked to elevated IL-6 concentration in young nonpregnant women. These findings represent elements of a nonpregnancy phenotype that parallels the findings observed in preeclampsia and in women at risk for ischemic cardiovascular disease. This suggests that the relationships observed during preeclampsia, which have been associated with placental pathology, may predate pregnancy and be independent of placental activity.
Similar content being viewed by others
Change history
30 December 2014
In the above-referenced article, Dr. Reem M. Sallam, MBBS, MSc, PhD, should have appeared as the fourth author, for significant contributions to project design and conducting critical aspects of the research.
The corrected reference for the article is:
Bernstein, IM, Damron, D, Schonberg, AL, Sallam, RM, Shapiro, R. The relationship of plasma volume, sympathetic tone, and proinflammatory cytokines in young healthy nonpregnant women. Reprod Sci. 2009;16;980–985.
Dr. Sallam’s affiliation at the time of the study was with the Department of Pathology, University of Vermont College of Medicine, Burlington, VT, USA. Currently, her affiliations are with the Clinical Chemistry Unit of the Department of Pathology, and the Obesity Research Center, King Saud University College of Medicine, Riyadh, Saudi Arabia; and the Department of Medical Biochemistry and Molecular Biology, Ain Shams University Faculty of Medicine, Cairo, Egypt.
References
ACOG Practice Bulletin. Diagnosis and management of preeclampsia and eclampsia. No. 33, January 2002.
Gallery ED, Hunyor SN, Gyory AZ. Plasma volume contraction: a significant factor in both pregnancy-associated hypertension (preeclampsia) and chronic hypertension in pregnancy. Quart J Med. 1979;48(192):593–602.
Soffronoff EC, Kaufmann BM, Connaughton JF. Intravascular volume determinations and fetal outcome in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 1977;127(1):4–9.
Silver HM, Seebeck M, Carlson R. Comparison of total blood volume in normal, preeclamptic and non-proteinuric gestational hypertensive disease by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol. 1998;179(1):87–93.
Yang CC, Chao TC, Kuo TB, Yin CS, Chen HI. Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. Am J Physiol. 2000;278(4):H1269–H1273.
Lewinsky RM, Riskin-Mashiah S. Autonomic imbalance in preeclampsia: evidence for increased sympathetic tone in response to the supine-pressor test. Obstet Gynecol. 1999; 91(6):935–939.
Davey DA, Macnab MF. Plasma adrenaline, noradrenaline, and dopamine in pregnancy hypertension. Br J Obstet Gynecol. 1981;88(6):611–618.
Schobel HP, Fischer T, Heuszar K, Geiger H, Schmieder RE. Preeclampsia—a state of sympathetic over-activity. New Engl J Med. 1996;335(20):1480–1485.
Kupferminc MJ, Peaceman AM, Aderka D, Wallach D, Socol ML. Soluble tumor necrosis factor receptors and interleukin-6 levels in patients with severe preeclampsia. Obstet Gynecol. 1996;88(3):420–427.
Benyo DF, Smarason A, Redman CWG, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001; 86(6):2505–2512.
Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R989–R990.
Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74(10):772–776.
Walraven CV, Mamdani M, Cohn A, Katib Y, Walker M, Rodger MA. Risk of subsequent thromboembolism for patients with preeclampsia. BMJ. 2003;326(7393):791–792.
Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive disorders of pregnancy and risk of hypertension and stroke in later life: results from a cohort study. BMJ. 2003; 326(7394):845–851.
Danesch J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary artery disease. JAMA. 1998;279(18):1477–1482.
Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. New Engl J Med. 2005;352(1):29–38.
Bernstein IM, Meyer MC, Osol G, Ward K. Intolerance to volume expansion: A theorized mechanism for the development of preeclampsia. Obstet Gynecol. 1998;92(2):306–308.
Damron DP, Bouchard BA, Shapiro RE, Schonberg AL, Bernstein IM. Platelet activation, sympathetic tone, and plasma volume in nulligravid women of reproductive age. Obstet Gynecol. 2004;103(5 pt 1):931–936.
Bernstein IM, Shapiro R, Whitsel A, Schonberg A. Relationship of plasma volume to sympathetic tone in nulliparous women. Am J Obstet Gynecol. 2003;188(4):938–942.
Vorchheimer DA, Becker R. Platelets in atherothrombosis. Mayo Clin Proc. 2006;81(1):59–68.
Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation. 1990;81(1):1–13.
Aardenburg R, Spaanderman MEA, Ekhart TH, van Eijndhoven HW, van der Hrijden OWH, Peeters LLH. Low plasma volume following pregnancy complicated by preeclampsia predisposes for hypertensive disease in a next pregnancy. BJOG. 2003;110(11):1001–1006.
Spaanderman MEA, Aardenburg R, Ekhart THA, et al. Non-pregnant circulatory volume status predicts subsequent pregnancy outcome in normotensive thrombophilic formerly preeclamptic women. Eur J Obstet Gynecol Reprod Med. 2001; 95(2):218–221.
Aardenburg R, Spaanderman ME, van Eijndhoven HW, de Leeuw PW, Peeters LL. A low plasma volume in formerly preeclamptic women predisposes to the recurrence of hypertensive complications in the next pregnancy. J Soc Gynecol Investig. 2006;13(8):598–603.
Copeland KC, Kenney FA, Sreekumaran Nair K. Heated dorsal hand vein sampling for metabolic studies: a reappraisal. Am J Physiol. 1992;263(5 pt 1):E1010–E1014.
Jiang NS, Machacek D, Wadel OP. Further study on the two-column plasma catecholamine assay. Mayo Clin Proc. 1976;51(2):112–116.
El-Sayed H, Goodal SR, Hainsworth R. Reevaluation of Evans blue dye dilution method of plasma volume measurement. Clin Lab Haematol. 1995;17(2):189–194.
Bernstein IM, Ziegler W, Stirewalt WS, Brumsted J, Ward K. Angiotensinogen genotype and plasma volume in nulligravid women. Obstet Gynecol. 1998;92(2):171–173.
Denq JC, O’Brien PC, Low PA. Normative data on phases of the Valsalva maneuver. J Clin Neuro. 1998;15(6):535–540.
Tjoa ML, van Vogt JMG, Blankenstein MA, Oudejans CBM, van Wijk IJ. Elevated C-reactive protein levels during first trimester of pregnancy are indicative of preeclampsia and intrauterine growth restriction. J Reprod Immunol. 2003;59(1):29–37.
Courtar DA, Spaanderman ME, Aardenburg R, Janssen BJ, Peeters LL. Low plasma volume coincides with sympathetic hyperactivity and reduced baroreflex sensitivity in formerly preeclamptic patients. J Soc Gynecol Investig. 2006;13(1):48–52.
Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary artery disease: meta-analyses of prospective studies. JAMA. 1998;279(18):1477–1482.
Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112(1):25–31.
Tracy RP. Inflammation, the metabolic syndrome and cardiovascular risk. Int J Clin Pract Suppl. 2003;(134):10–17.
Zieske AW, Tracy RP, McMahan CA, et al;. Pathobiological Determinants of Atherosclerosis in Youth Research Group. Elevated serum C-reactive protein levels and advanced atherosclerosis in youth. Arterioscler Thromb Vasc Biol. 2005; 25(6):1237–1243.
Kamat SG, Kleiman NS. Platelets and platelet inhibitors in acute myocardial infarction. Cardiology Clin. 1995;13(3):435–447.
Schomig A, Richardt G. Cardiac sympathetic activity in myocardial ischemia: release and effects of noradrenaline. Basic Res Cardiol. 1990;85(suppl 1):9–30.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by NIH R0–1 HL 71944 and GCRC MO-1 RR109.
Rights and permissions
About this article
Cite this article
Bernstein, I.M., Damron, D., Schonberg, A.L. et al. The Relationship of Plasma Volume, Sympathetic Tone, and Proinflammatory Cytokines in Young Healthy Nonpregnant Women. Reprod. Sci. 16, 980–985 (2009). https://doi.org/10.1177/1933719109338876
Published:
Issue Date:
DOI: https://doi.org/10.1177/1933719109338876