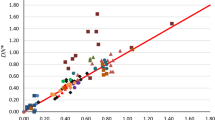
The UQCISD(T)/6-31g(d)//UB3LYP/6-31g(d) method and PCM solvation model were used to study the solvent effects on radical clock reactions. The solvents included cyclohexane, benzene, tetrahydrofuran, methylene chloride, acetone, methanol, acetonitrile, dimethylsulfoxide, nitromethane and water. We found very small solvent effects on the rearrangement activation free energy of cyclobutylmethyl and 1-hexen-6-yl radicals. Therefore, it is valid to use a calibrated radical clock in an unclear reaction medium because the speed of the radical clock should not change significantly by the solvent effect. In addition, we separated the solvent effects on radical rearrangement into three components, electrostatic, cavitation and dispersion/repulsion. We discussed the contribution of each component in detail.
Similar content being viewed by others
Rights and permissions
About this article
Cite this article
Fu, Y., Li, RQ., Liu, L. et al. Solvent effect is not significant for the speed of a radical clock. Res Chem Intermediat 30, 279–286 (2004). https://doi.org/10.1163/156856704323034012
Published:
Issue Date:
DOI: https://doi.org/10.1163/156856704323034012