Abstract
The investigation of novel electrolytes for lithium metal anodes has been conducted. Incorporation of LiNO3 into lithium difluoro(oxalato) borate (LiDFOB) in ethylene carbonate (EC) and dimethyl carbonate (DMC) electrolytes results in a significant improvement in capacity retention and Coulombic efficiency. While the solubility of LiNO3 is very low in common carbonate solvents (∼0.03 M), the use of triethyl phosphate (TEP) significantly increases the solubility of LiNO3 and improves the capacity retention and Coluombic efficiency of lithium metal anodes. Ex-situ surface analysis of the cycled electrodes suggests that incorporation of LiNO3 results in nitrogen containing species (NO3−, NO2−, and N3−) in the solid electrolyte interphase (SEI) which is likely responsible for the performance enhancement.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Pursuit of the application of lithium metal as an anode material for the next generation lithium battery technology has received significant recent attention.1–4 The importance derives from the fundamental properties of lithium metal anodes, which have a very high theoretical specific capacity of 3,860 mAh g−1, extremely low negative potential (−3.04 V vs. standard hydrogen electrode) and a low gravimetric density of 0.534 g cm−3. However, several barriers exist in commercializing lithium metal anodes, including the formation of lithium dendrites, safety risks caused by dendritic lithium, and low Coulombic efficiency, especially in commercial carbonate electrolytes. Nevertheless, the development of carbonate electrolytes for lithium metal anodes is desired, given their versatile properties, such as a high dielectric constant, chemical stability, and wide electrochemical window.5
The preferential decomposition of electrolyte components generates a Solid Electrolyte Interphase (SEI), which can be modified to improve the cycling performance of lithium metal anodes.6 In particular, lithium nitrate (LiNO3) has been observed to generate a beneficial SEI for several anode materials, including lithium metal. For example, it has been demonstrated that LiNO3 is an exceptional additive in ether electrolytes for the Li metal anode in lithium-sulfur batteries.7,8 In addition, Nguyen et al. previously reported that the performance of silicon-graphite anodes can be improved upon incorporation of EC electrolytes saturated with lithium nitrate, estimated to be ∼0.09 M LiNO3 dissolved in EC:DEC (1:1, by mass).9 Moreover, solubility-mediated sustained release of LiNO3 in carbonate electrolytes has been shown to improve the stability of the lithium metal anode.10 Therefore, exploring the limits of saturating LiNO3 in carbonate electrolytes is of interest for improving the performance of lithium metal anodes.
In addition, there has been recent interest in the use of phosphate solvents in lithium battery electrolytes.11 While phosphate containing electrolytes have been previously investigated as flame retarding co-solvents, the incorporation of phosphates was typically reported to result in poor SEI stability and thus poor cycle life.12 However, the recent investigations revealed that the good solvation properties of organophosphates can enable the dissolution of high concentrations of lithium salts and lead to excellent cycling performance. Other recent investigations of lithium metal anodes suggest that the lithium salt in the electrolyte has a very strong influence on the cyclability of lithium metal anodes.13,14 One of the best salts for lithium metal anodes is lithium difluoro(oxalato)borate (LiDFOB).15,16 The results suggest an importance of the relative reduction reactions of the solvents and the salts in SEI formation and stability.17,18 These results lead to the investigation of electrolytes containing LiDFOB, LiNO3 and organophosphates.
The performance of lithium metal electrodes cycled with lithium difluoro(oxalato)borate (LiDFOB), lithium nitrate (LiNO3) and triethyl phosphate (TEP)-containing carbonate electrolytes has been investigated with Cu||LiFePO4 cells. The in-situ formation of lithium metal and low reactivity of LiFePO4 in Cu||LiFePO4 cells ensure that the electrolyte components do not react with the electrode surfaces prior to the initial lithium plating cycle, as previously reported.19 The LiNO3 additive was observed to improve the cycling performance of Cu||LiFePO4 cells with carbonate electrolytes. Further, TEP was observed to increase the solubility of LiNO3 in carbonate electrolytes, leading to significant improvement of the cycling performance of Cu|| LiFePO4 cells. By analyzing the surface of lithium metal plated with the investigated electrolytes with X-ray Photoelectron Spectroscopy (XPS), it is suggested that the improved cycling performance derives from LiNO3 decomposing to modify the SEI of the lithium metal anode.
Experimental
Electrochemistry
Electrochemical characterization was performed using Cu||LiFePO4 2032 coin cells. The Cu||LiFePO4 cells were assembled with a Cu metal foil negative electrode (15 mm diameter, MTI Corporation), two Celgard 2400 separators (19 mm diameter), and a LiFePO4 positive electrode (91% active material, 13.7 mm diameter, 83 μm thickness, 12 mg/cm2 loading, MTI corporation), the other 9% of the composite electrode is composed of conductive carbon and PVDF coated on aluminum. The Cu||LiFePO4 and Li||Li cells were prepared with 40 μL/cm2 of electrolyte. Electrolytes investigated include 1.0 M LiDFOB in ethylene carbonate: dimethyl carbonate (16.8:83.2, volume:volume, EC:DMC, LiDFOB EC electrolyte), 1.0 M LiDFOB in EC:DMC with saturated LiNO3 (estimated 0.03 M LiNO3 in 16.8:83.2, EC:DMC, LiDFOB LiNO3 EC electrolyte), 1.0 M LiDFOB in triethyl phosphate (TEP):EC:DMC (8.4:8.4:83.2, by volume, LiDFOB TEP:EC electrolyte), and 1.0 M LiDFOB in TEP:EC:DMC with saturated LiNO3 (estimated 0.2 M in 8.4:8.4:83.2, by volume, LiDFOB LiNO3 TEP:EC electrolyte). The LiDFOB, EC, and DMC electrolyte components were battery grade and supplied from Gotion Inc. The LiNO3 (99.99%, trace metals basis) and TEP (≥99.8%, dried over molecular sieves) were purchased from Sigma-Aldrich. The copper metal foil was sonicated with isopropanol (2 × 2 minutes), punched to the specified diameter, and dried at 110°C, overnight under vacuum prior to cell assembly. The LiFePO4 electrodes were punched to the specified diameter, and dried at 110°C overnight under vacuum prior to cell assembly. The cycling procedure consisted of plating Li metal at 0.1 mA/cm2 (approx. C/20 rate, where C represents the theoretical capacity of LiFePO4) with subsequent stripping and plating at 0.4 mA/cm2 (approx. C/4 rate), within a voltage window of 2.0 – 4.0 V, using an Arbin BT2000 battery cycler at 25°C. There was a rest period of one hour between cell construction and the beginning of the electrochemical protocol.
XPS
XPS measurements were acquired with a K-alpha Thermo system using Al Kα radiation (hν = 1486.6 eV) under ultra-high vacuum (˂1 × 10−12 atm) and a measured spot size of 400 μm in diameter. Lithium metal was deposited onto Cu foil according to the first charge procedure outlined in the electrochemistry section (charge to 4.0 V at C/20 rate), and held at rest for approximately 4 hours to ensure cell equilibration before disassembly. Electrodes were washed with 4 × 500 μL battery grade DMC and dried under vacuum for 10 minutes, then overnight in the argon glovebox. The samples were transferred from the argon glove box in an air-free transfer case, while sealed under vacuum. The binding energy was corrected based on the F1s spectrum, assigning LiF to 685 eV.
Results
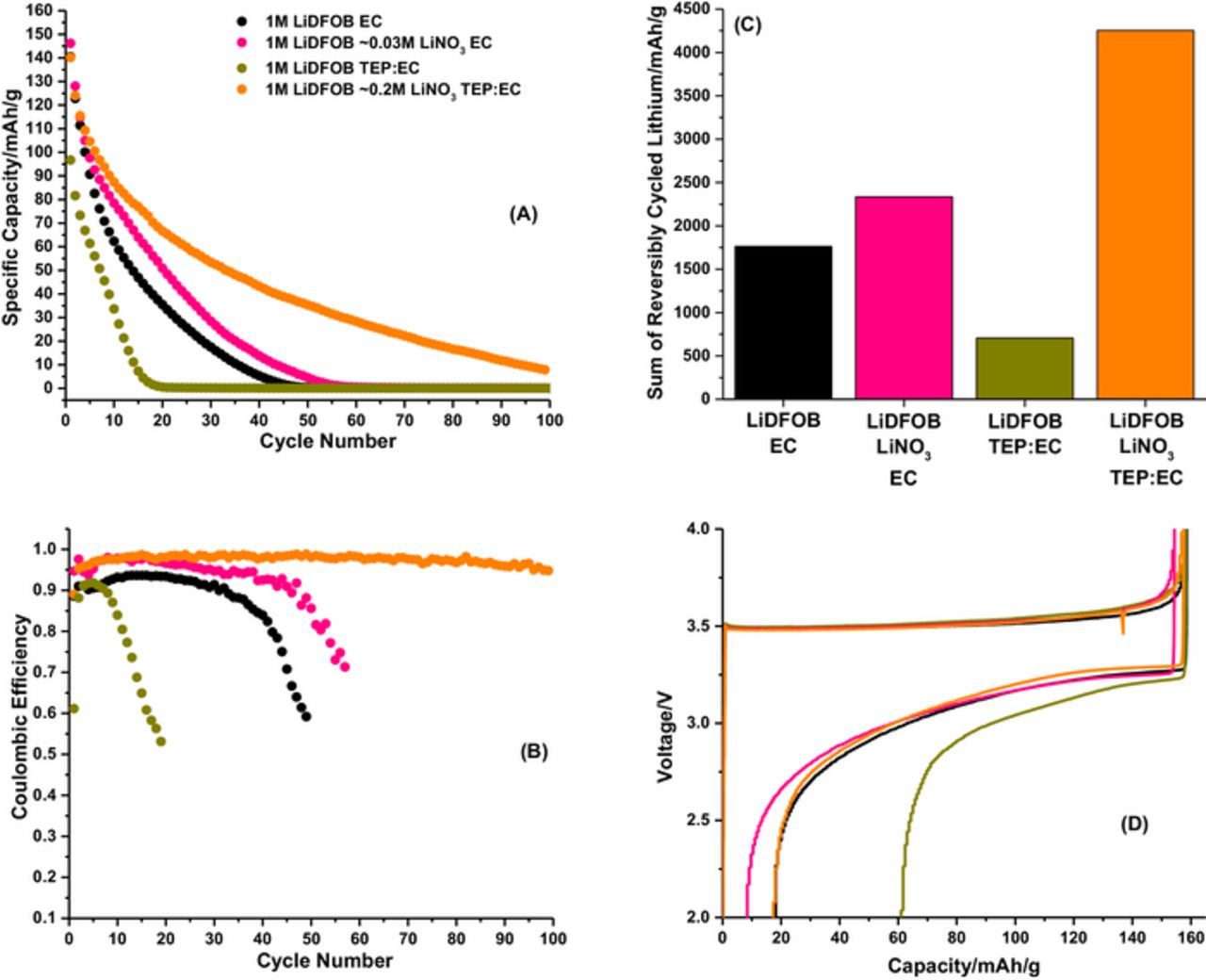
The stripping capacity vs. cycle number and Coulombic efficiency vs. cycle number are provided in Figures 1A and 1B, respectively, for the electrolytes investigated. For this investigation, a baseline carbonate electrolyte, 1 M lithium difluoro(oxalate)borate (LiDFOB) dissolved in ethylene carbonate (EC): dimethyl carbonate (DMC) has been selected (LiDFOB EC electrolyte, see electrolyte abbreviations in experimental section). The cell containing the LiDFOB EC electrolyte achieves over 25 cycles before dropping below 20% initial capacity (Fig. 1A) and a first cycle Coulombic efficiency of 89% (Fig. 1B) is observed for the lithium metal anode, as previously reported.20 Upon saturating the LiDFOB EC electrolyte with LiNO3 (LiDFOB LiNO3 EC electrolyte) the Coulombic efficiencies are further improved to 98% after 10 cycles (Fig. 1B) along with an improvement in cycle lifetime. Furthermore, employing TEP to increase the solubility of LiNO3 in the electrolyte to ∼0.2 M (LiDFOB LiNO3 TEP:EC) leads to further improved performance, with efficiencies reaching up to 99% after 15 cycles (Fig. 1B) and cycling for over 65 cycles before the capacity drops below 20% of the initial capacity (Fig. 1A). The LiDFOB TEP:EC electrolyte without LiNO3 has very poor performance compared to the other electrolytes investigated with a first cycle Coulombic efficiency of 62% (Fig. 1B) and lasting only 13 cycles before dropping below 20% of the initial capacity (Fig. 1A). Therefore, the improved performance for the LiDFOB LiNO3 TEP:EC electrolyte is due to the increased concentration of LiNO3 in the electrolyte, and not the presence of TEP. The improved performance observed with the LiDFOB LiNO3 TEP:EC electrolyte is further illustrated in Figure 1C, with the sum of reversibly cycled lithium over the first 100 cycles reaching over 4250 mAh/g, almost twice the amount observed for the next best performing LiDFOB LiNO3 EC electrolyte. It is worth noting the sum of reversibly cycled lithium for the LiDFOB LiNO3 TEP:EC electrolyte surpasses the previously reported performance for FEC electrolytes.20 Finally, the first cycle galvanostatic cycling profile (Fig. 1D) eludes to the dramatic decrease in cycling performance for the LiDFOB TEP:EC electrolyte. Specifically, a decrease in discharge voltage is observed upon stripping lithium for the LiDFOB TEP:EC electrolyte, in comparison to the other electrolytes, suggesting a more resistive SEI is formed on the lithium metal anode with the LiDFOB TEP:EC electrolyte.
Figure 1. Stripping capacity vs. cycle number (A), Coulombic efficiency vs. cycle number (B), sum of reversibly cycled lithium (C), and the first cycle galvanostatic cycling profile (D) for the electrolytes investigated in Cu||LiFePO4 cells.
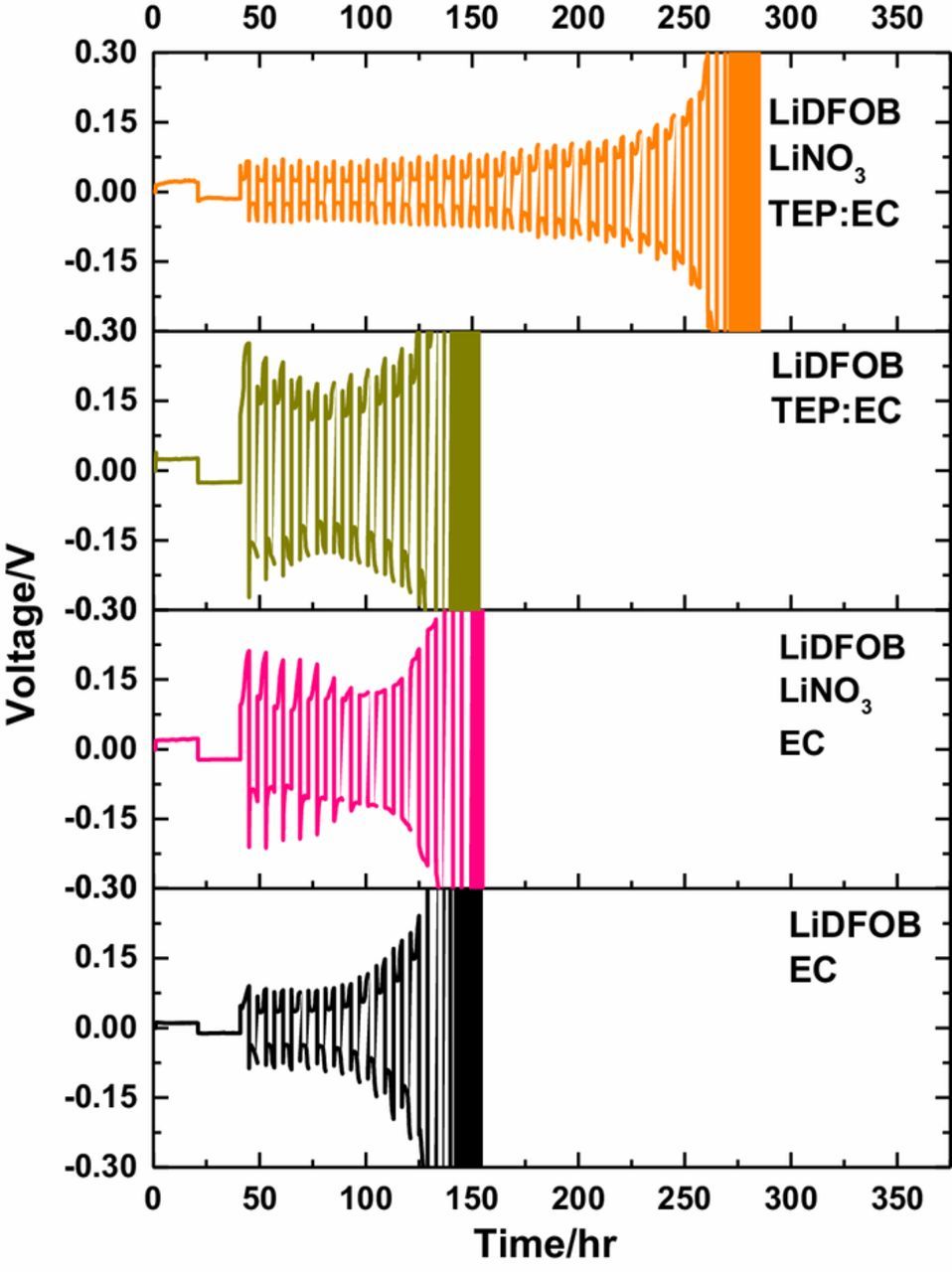
The electrochemistry of these electrolytes was also characterized in Li||Li symmetric cells, shown in Figure 2. The cycling performance of cells with LiDFOB EC, LiDFOB TEP:EC and LiDFOB LiNO3 EC electrolyte are similar. The high reactivity of lithium metal in electrodes makes it challenging to resolve the minor changes in electrolyte performance, as electrolyte components are likely consumed at a higher rate than in the lower reactivity Cu||LiFePO4 cells. However, it is clear that the LiDFOB LiNO3 TEP:EC has the best performance, with cycle lifetime almost twice that observed for the other electrolytes, consistent with the improvement observed in Cu||LiFePO4 cells (Figure 1C). These observations confirm that increasing the concentration of LiNO3 via dissolution in a good solvent such as TEP improves the cycling performance of the lithium metal anode.
Figure 2. Li||Li symmetric cells cycled with the investigated electrolytes.
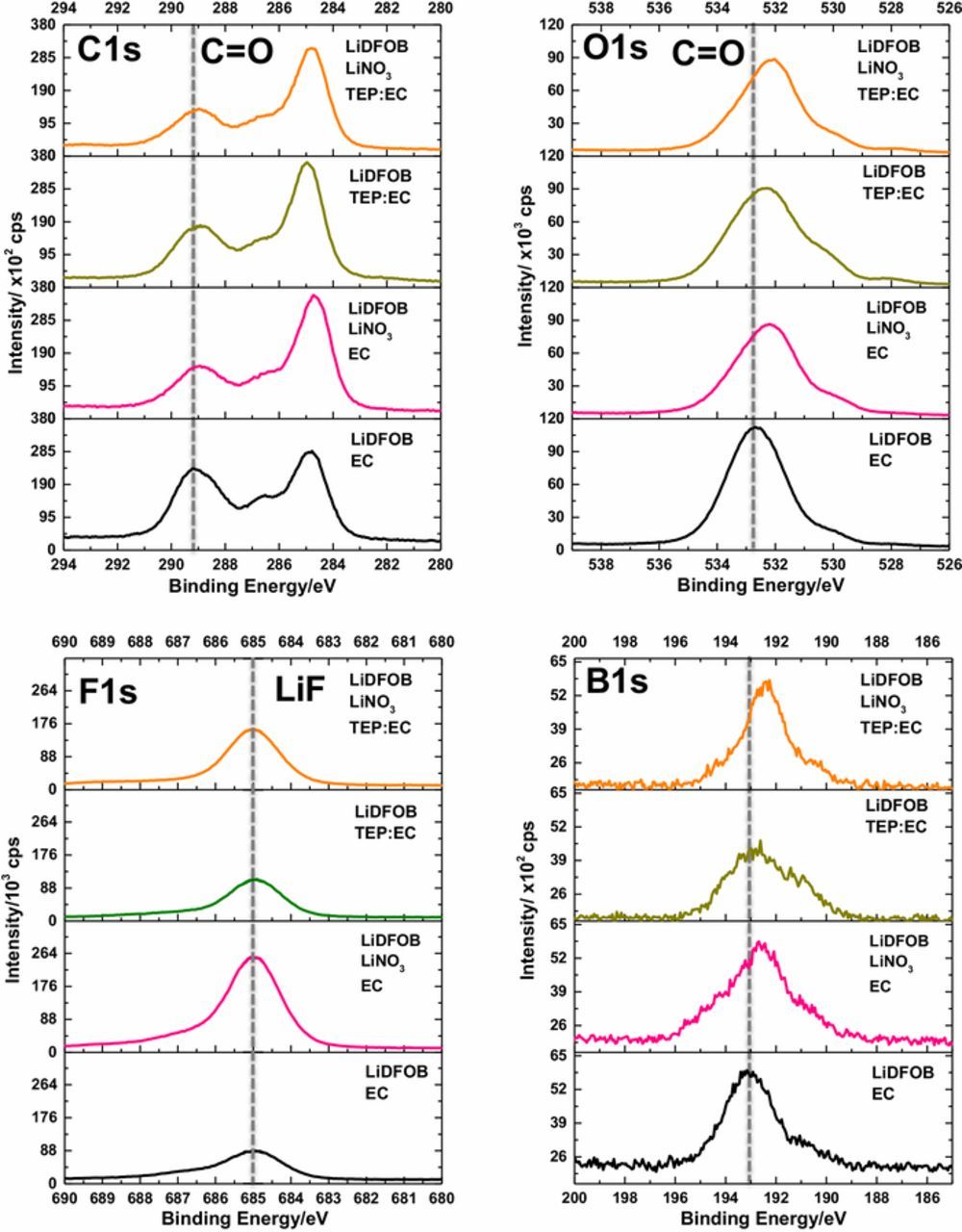
The C1s, O1s, F1s, and B1s spectra of lithium metal plated with the investigated electrolytes are plotted in Figure 3. In general, these spectra are dominated by the decomposition products of LiDFOB. For example, in the C1s spectra, peaks are observed at 289.1 eV (C=O), and 286.6 eV (C-O), with the greatest relative intensity observed for lithium plated from the baseline LiDFOB EC electrolyte, suggesting the formation of oxalate species, consistent with previous work.15,20 Lower intensities for these peaks are observed for lithium plated with the other electrolytes, suggesting that LiDFOB reduction is inhibited when LiNO3 or TEP is present in the electrolyte. The same trend is apparent in the O1s spectra, where a broad peak at ∼532.5 eV (mixture of C=O/C-O) is most intense for lithium plated with the baseline LiDFOB EC electrolyte.15,20 As observed in the F1s spectra, LiF (685.0 eV) is the dominant product for lithium plated with each electrolyte, consistent with previous results. The LiF intensity is weakest for lithium plated with the baseline LiDFOB electrolyte, as it likely has the thickest layer of oxalate species on the upper surface of the SEI.20 Finally, a broad peak is observed at ∼193.0 eV in the B1s spectra for lithium plated with each electrolyte, consistent with LiDFOB decomposition.15,20
Figure 3. C1s, O1s, F1s, and B1s spectra of lithium metal plated on the first cycle with the investigated electrolytes.
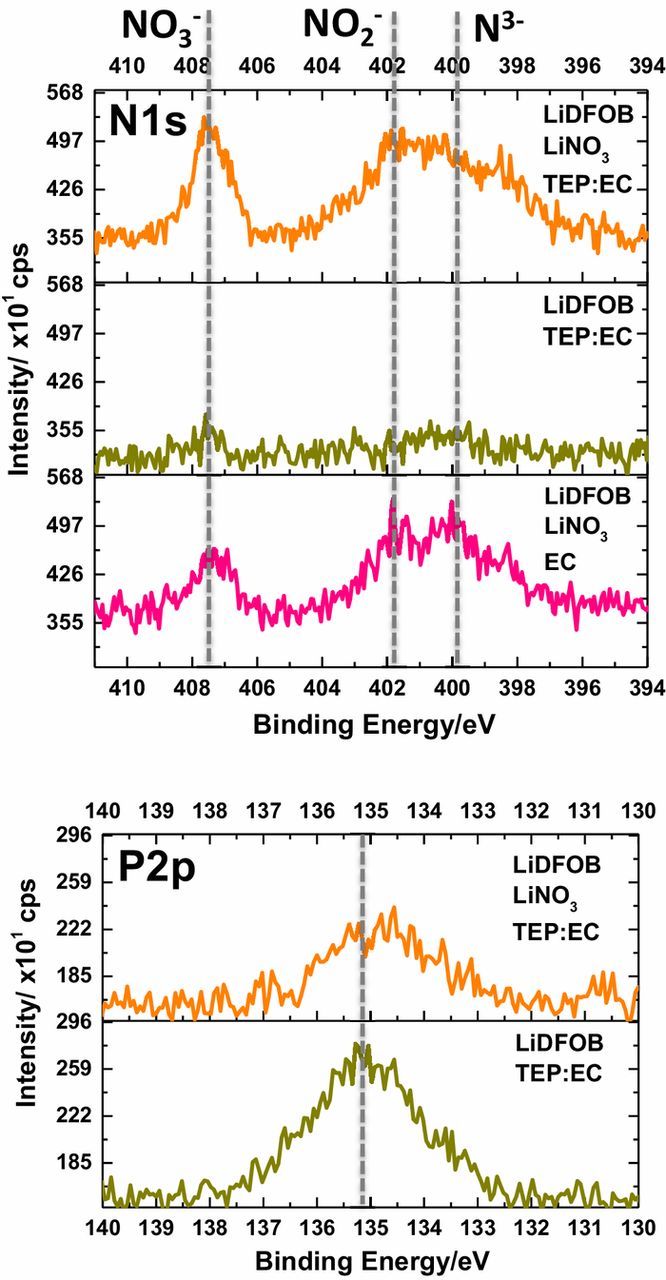
The N1s and P2p spectra for lithium metal plated with the investigated electrolytes are plotted in Figure 4. Lithium plated with the electrolytes containing LiNO3 contain the expected decomposition products including NO3− (∼407.5 eV), NO2− (∼402.0 eV), and N3−(∼400.0 eV) as observed in the N1s spectra.9,10 These products are not observed for lithium plated with the LiDFOB TEP:EC electrolyte. These observations suggest the incorporation of LiNO3 decomposition products in the SEI are beneficial for improving the performance of the lithium metal anode. Further, there is a broad peak observed at ∼135.0 eV in the P2p spectra for lithium plated with both electrolytes containing TEP, consistent with reduction of TEP. There appears to be a significant amount of TEP decomposition for the LiDFOB TEP:EC electrolyte, suggesting that TEP decomposition is detrimental to the cycling performance of the lithium metal anode, since these cells have the poorest cycling performance. Apparently, saturating TEP with LiNO3 can overcome the negative impact of TEP on the cycling performance of the lithium metal anode, perhaps through a similar mechanism as described for concentrated lithium bis(fluorosulfonyl)amide/trimethyl phosphate electrolytes cycled with graphite anodes.11 The improved performance may be due to synergistic reactions of LiNO3 and TEP to generate SEI products similar to that of the LiPON solid state electrolyte.21 However, the increased concentration of LiNO3 in the electrolyte does not significantly change the composition of the SEI for the lithium metal anode compared to electrolytes with lower concentrations of LiNO3. It is likely that LiNO3 is continuously consumed during cycling and the increased concentration of LiNO3 provides a larger supply of LiNO3, increasing the number of cycles that generate an SEI rich in LiNO3 decomposition products. Similar results have been reported with FEC electrolytes and silicon based anode materials.22,23 Furthermore, recent work has demonstrated that LiNO3 and TEP containing electrolytes can be used in high voltage lithium metal batteries.24 However, a more detailed investigation is required to explore these mechanisms. In general, LiNO3 appears to preferentially react on the surface of lithium metal, modifying the SEI, and thereby improving the cycling performance of the lithium metal anode.
Figure 4. N1s and P2p spectra of lithium metal plated on the first cycle with the investigated electrolytes.
Conclusions
Developing carbonate electrolytes for the lithium metal anode is important for enabling high-energy lithium batteries. Employing phosphate solvents to increase the solubility of beneficial additives, such as LiNO3, can improve the electrochemical performance of the lithium metal anode significantly. For example, by adding a saturated solution of LiNO3 in TEP to a LiDFOB-based carbonate electrolyte, it was possible to cycle more than double the amount of lithium than was achieved with the original LiDFOB-based carbonate electrolyte. This observation motivates researchers to pursue various new solvents, compatible with carbonate solvents, to increase solubility of desired additives that are sparingly soluble in pure carbonate solvents.
ORCID
Zachary L. Brown 0000-0003-0772-3159
Satu Heiskanen 0000-0001-9812-0147
Brett L. Lucht 0000-0002-4660-0840