Abstract
A new "hot formation" protocol is proposed to improve lower temperature cycling of lithium metal batteries. The cycling stability of anode-free pouch cells under low pressure (75 kPa) is shown to decline significantly as the cycling temperature is decreased from 40°C to 20°C. At low pressure and 40°C the initial morphology of the lithium anode is dense and columnar, far superior to that plated at 20°C. For "hot formation" two initial 40°C cycles (C/10 charge C/2 discharge) are conducted prior to extended low temperature (20°C) cycling. These two initial cycles have a surprisingly large impact; capacity retention to 80% is increased from only 18 cycles without hot formation to 60 cycles with hot formation at low pressure. When the applied pressure is increased to 1200 kPa, the hot formation (20°C cycling) cells show 85% capacity retention at 100 cycles. The benefits established during these two initial formation cycles are apparently carried forward to improve the longer term performance of lithium metal cells tested at room temperature.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Replacing the graphite anode with lithium metal is considered a critical step on the roadmap to improve energy density and develop next-generation batteries.1–3 Despite this consensus, no one is exactly sure how practical, safe, long-lived lithium metal cells will actually be developed. Cycling lithium metal in liquid electrolytes often results in high surface area "mossy" or "dendritic" morphologies which lead to rapid cell failure and safety concerns.4,5 The cycle life of these liquid cells is particularly short when using the limited quantities of excess lithium required for high energy density batteries.6,7 While solid state electrolytes can potentially offer improved cycling stability and safety, the development of a solid electrolyte chemistry satisfying the myriad of physical, chemical, and economic criteria required for commercialization remains elusive.8–10
In addition to developing lithium metal cells with good cycling stability and safety, these cells must also match or exceed the operating windows of lithium-ion cells. For instance, the United States Advanced Battery Consortium (USABC) has published a number of EV battery performance goals including a desired operating temperature range of −30 to 52°C.11 The challenge of achieving adequate low temperature conductivity in solid electrolytes is well documented.10,12,13 However, the variation in the mechanical properties of lithium with temperature can present potential issues for the cycling of both liquid and solid electrolyte lithium metal batteries across a range of temperatures. Lithium has a relatively low melting point (453 K) so there is noticeable variation in its mechanical properties over the desired operating temperature range of EV batteries.14 Micro-pillar compression experiments have revealed the strength of lithium has both a strong size and temperature dependence; yield strength was shown to increase with decreasing micro-pillar size and decreasing temperature.15 Studies have also shown that lithium undergoes creep deformation at battery relevant temperatures.11,14 Dasgupta has demonstrated that this creep stress increases as temperature decreases, that is, lithium becomes more difficult to deform at lower temperatures.14 These studies provided valuable information on the mechanical properties of lithium under battery relevant conditions and allude to the importance of these factors for battery operation, but no cells were cycled to determine the effect on battery performance.
A few recent studies have probed the effect of temperature on lithium cycling through an analysis of lithium metal coin cells with liquid electrolytes. These studies found superior plating morphology and cycling stability at high temperature (60°C) compared to room temperature.16,17 This was attributed to an improved lithium diffusion coefficient in the electrolyte as well as more favorable solid electrolyte interface (SEI) morphology at higher temperature. There was no mention of the varying mechanical properties of lithium at higher temperature although this also may have been a factor. This improved capacity retention at high temperature demonstrates how the lithium metal system is quite different from conventional lithium-ion cells where higher temperatures result in accelerated cell failure due the increased rate of parasitic reactions.18 Additionally, lithium metal cells can be quite sensitive to chemical cross-talk, that is, products of reactions at the cathode affecting lithium plating at the anode.19,20 Changing the cell operating temperature will change the rate of these cross-talk reactions, which may also affect cell performance. Due to these complex considerations more detailed investigations regarding the impact of temperature on the operation of lithium metal batteries are required.
In this study we investigate the effect of temperature on the performance of NMC 532 || Cu anode-free lithium metal pouch cells with a lithium difluoro(oxalato)borate (LiDFOB)/LiBF4 dual salt electrolyte21 optimized for lithium metal cycling. These cells were tested at low (20°C) and high (40°C) temperature conditions as well as low (75 kPa) and high (1200 kPA) pressure conditions to gauge the potential pressure-temperature interaction. Based on insights from this analysis an initial high temperature or "hot formation" protocol is proposed which can improve subsequent low temperature cycling performance of lithium metal cells. The efficacy of this hot formation procedure is analyzed with regard to its effect on cycling stability, lithium plating morphology, and the parasitic reactions occurring within the cell.
Experimental
Pouch cell preparation
Dry (no electrolyte) and sealed Li[Ni0.5Mn0.3Co0.2]O2 (NMC 532) || Cu pouch cells were obtained from Li-FUN Technology (Xinma Industry Zone, Golden Dragon Road, Tianyuan District, Zhuzhou City, Hunan Province, China, 412000). The NMC used in these pouch cells was single crystal NMC with a titanium based coating.22 The single side positive electrode loading was 16.1 mg cm−2 (50 μm) and the electrode consisted of 94% active material. Positive electrode area is 97.5 cm2. Assuming the positive electrode active material has a capacity of 200 mAh/g when charged to 4.5 V, these cells have a theoretical capacity of 290 mAh at 4.5 V. The measured C/2 discharge capacity from 4.5 V to 3.6 V on the first cycle after formation is 245 mAh at 40°C or 210–220 mAh at 20°C. Cells were transferred into an argon-filled glove box, opened, and dried at 100°C for 14 hours under vacuum.
Reagents used for electrolytes include fluoroethylene carbonate (FEC, BASF, purity 99.4%), diethyl carbonate (DEC, BASF, purity >99%), lithium difluoro(oxalato)borate (LiDFOB, Capchem, 99%), and lithium tetrafluoroborate (LiBF4, BASF, 99%). The electrolyte used for all cells tested was 0.6M LiDFOB 0.6M LiBF4 in FEC:DEC 1:2 v:v. unless otherwise specificed. Cells were filled with 0.5 mL of electrolyte (approximately 2g Ah−1), resealed, and removed from the glove box. The cells were held at 1.5 V for 24 hours at room temperature to ensure complete wetting and then transferred to a temperature controlled chamber for cycling.
Pouch cell cycling
Anode free pouch cells were cycled between 3.6 and 4.5 V with a C/5 charge rate and C/2 discharge rate in a temperature controlled environment. The "hot formation" protocol consisted of 2 initial cycles between 3.6 and 4.5 V with a C/10 charge rate and C/2 discharge rate at 40°C. The cells cycled at low applied pressure were clamped with rubber blocks inside a plastic enclosure (Figure S1a) which maintained a uniaxial pressure of approximately 75 kPa. Cells cycled under the high pressure condition were constrained in an aluminum enclosure (Figure S1b) with a uniaxial pressure of approximately 1200 kPa.
Scanning electron microscopy
Samples were analyzed on a Hitachi S-4700 Field Emission Scanning Electron Microscope in secondary electron imaging mode with an electron accelerating voltage of 3.0kV and working distance of approximately 12 mm. Lithium samples were rinsed with DMC and dried before being affixed to the SEM sample stub via carbon tape. The samples were transferred to the SEM in an argon-filled container and were briefly (< 10s) exposed to air during the final transfer into the SEM chamber.
Results
Anode free cell performance as a function of temperature
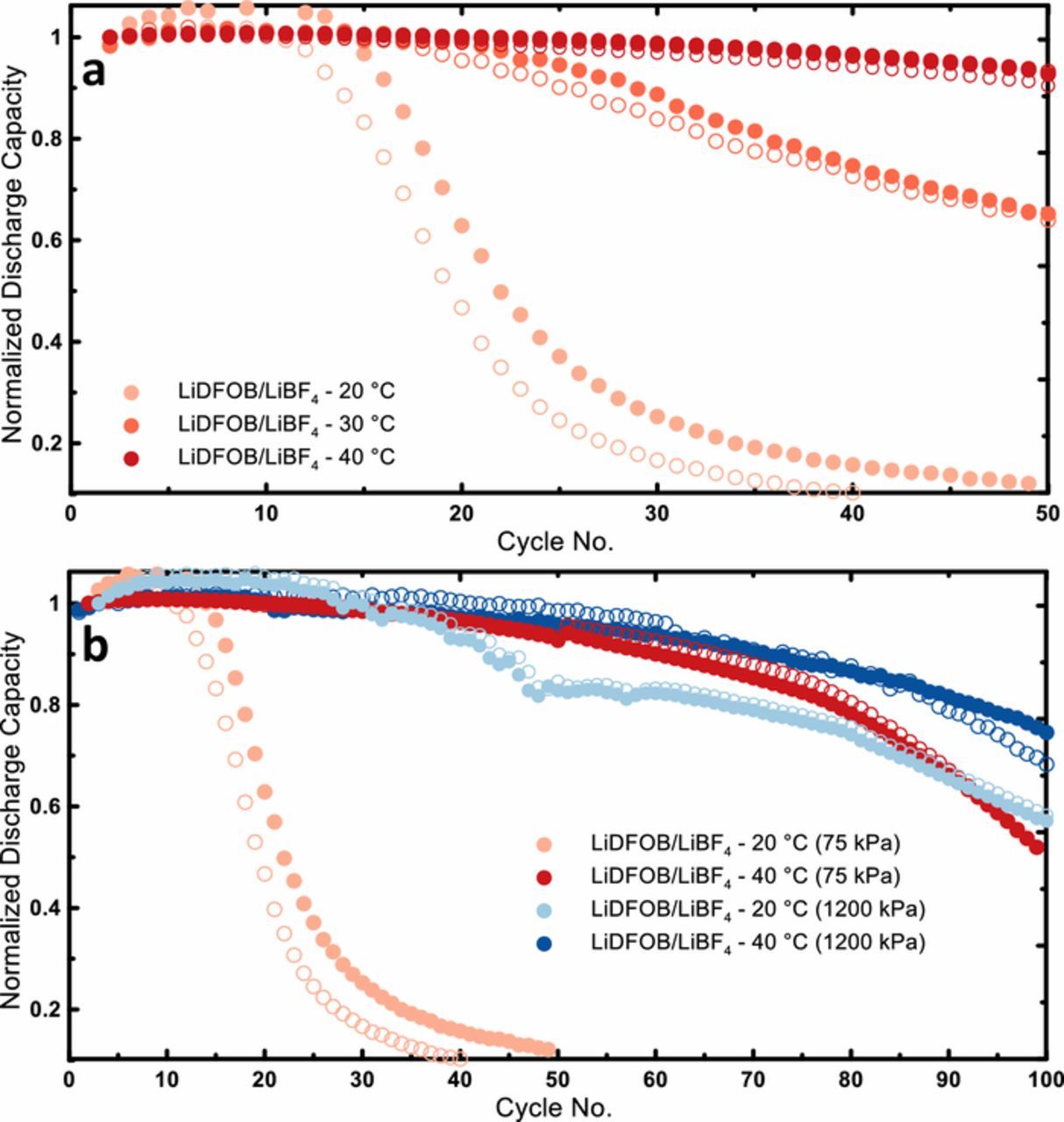
Figure 1a summarizes the capacity retention of identical NMC 532 || Cu pouch cells with LiDFOB/LiBF4 dual salt electrolyte cycling at varying temperatures. All cells were cycled with a C/5 charge rate and a C/2 discharge rate and constrained with low pressure (75 kPa) in a plastic enclosure. It's clear that the performance of these cells is a strong function of the cycling temperature. Replicates or brother cells run for each condition are represented by open and filled symbols of the same color. After 50 cycles at 40°C the anode-free cell has shown minimal fade and is still well above 80% capacity retention. However, when the temperature is reduced to 30°C this same cell falls below 80% retention at just over 30 cycles. If the temperature is reduced to 20°C, fade is further accelerated as the cell only achieves 18 cycles to 80% retention and is almost completely dead after just 50 cycles. Conventional lithium-ion cells generally demonstrate the opposite trend within this temperature range; higher temperatures lead to accelerated fade due to the increased rate of parasitic reactions that decrease coulombic efficiency. Why then does the performance of these anode free cells deteriorate so rapidly when the temperature is reduced? There are a number of factors to consider. Due to its low melting point the mechanical properties of lithium metal such as its yield stress and creep stress are strong functions of temperature. At higher temperatures lithium is softer and easier to deform so at 40°C the low pressure applied to the cells in Figure 1a may be enough to adequately constrain the lithium and delay the onset of mossy or dendritic growth. Additionally, there may be beneficial "cross-talk" reactions occurring within the cell producing "additives" that aid in lithium metal cycling; at lower temperatures the rates of these reactions would be reduced.
Figure 1. a) Normalized discharge capacity vs. cycle number for NMC 532 || Cu anode-free cells with 0.6M LiDFOB 0.6M LiBF4 FEC:DEC 1:2 electrolyte cycled at 20°C, 30°C, and 40°C; (b) Comparison of anode free discharge capacity retention at low (20°C) and high (40°C) temperature conditions as well as low (75 kPa) and high (1200 kPa) pressure conditions. Replicates or brother cells run for each condition are represented by open and filled symbols of the same color.
To first explore the role of the mechanical properties of lithium, the effect of mechanical pressure on anode-free cell performance was evaluated at high and low temperature. Figure 1b shows capacity versus cycle number for anode-free cells cycled at 20°C and 40°C under low pressure (75 kPa) along with identical cells cycled at the same temperatures but constrained at much higher pressure (1200 kPa). At 20°C, pressure has a significant impact on performance, the number of cycles to 80% retention increases from only 18 in the low pressure case to 50 under high pressure. The longer term fade rate is much reduced at high pressure as the 1200 kPa cell retains 60% capacity after 100 cycles compared to only 10% at 75 kPa. At 40°C, however, the higher pressure has a smaller impact with the 1200 kPa cell showing only a modest improvement over the one cycled at 75 kPa. This aligns with the concept of lithium being softer at higher temperatures and therefore needing higher pressures at lower temperature in order to be adequately constrained. However, the capacity retention of the cell under high pressure at 20°C is still worse than the cell cycled at 40°C with very little pressure. Normalized capacity is plotted here for clarity, but due to the lower cell impedance at higher temperature, the 40°C cell also has a larger capacity (cycling more lithium) and still shows better capacity retention than the 20°C cell under pressure. This suggests that pressure, while important, is not the only factor influencing the low temperature performance of these cells and additional measures need to be taken to improve the 20°C cycling behavior.
Hot formation of anode-free cells
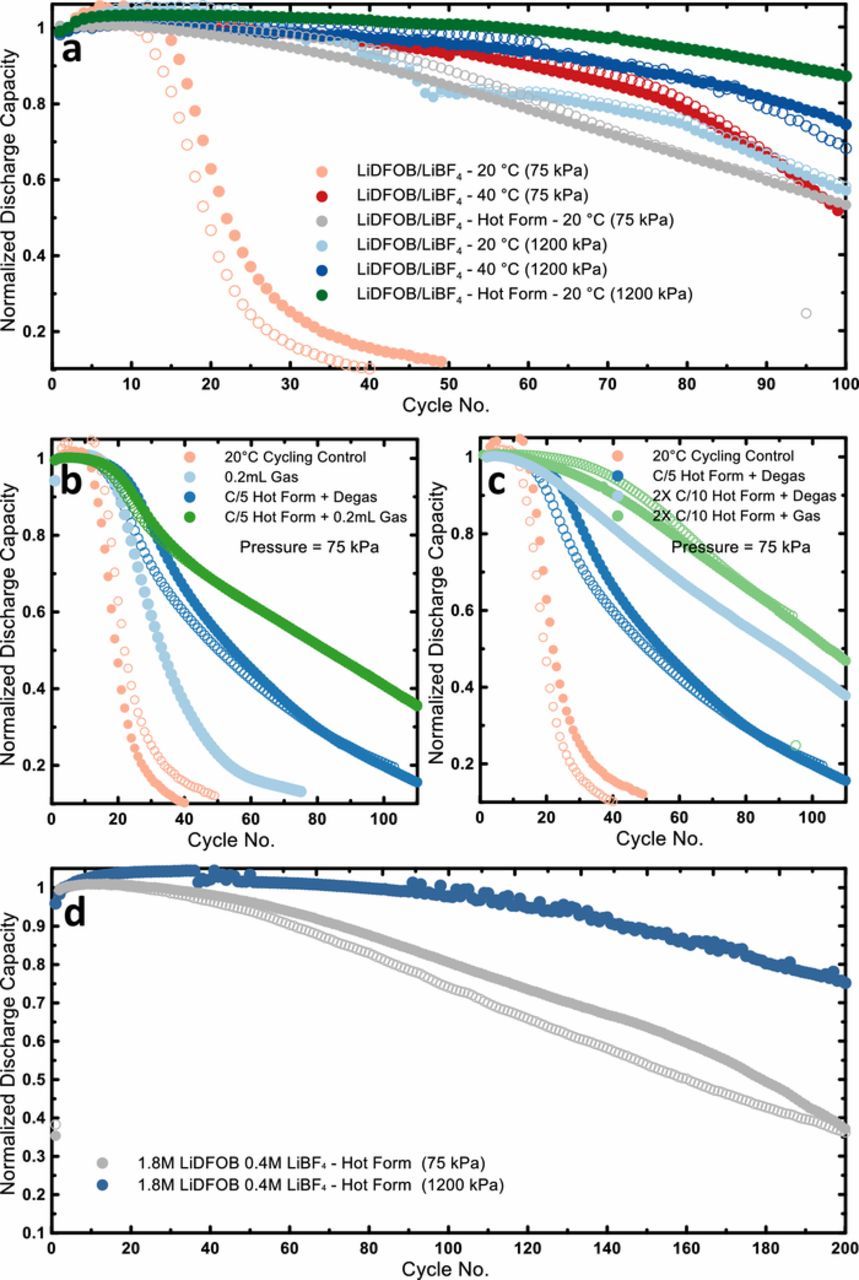
Initial formation cycles have been used in lithium metal cells previously, but these formation cycles occurred at the same temperature as the subsequent cycles.23 Due to the superior performance of our anode-free cells at higher temperature we wanted to investigate the impact of high temperature formation cycles on subsequent lower temperature cycling. Figure 2a shows the cycling behavior of dual salt anode-free cells which underwent two formation cycles at 40°C consisting of a C/10 charge and C/2 discharge followed by cycling (C/5 charge C/2 discharge) at 20°C, under low pressure. The cycling behavior of identical cells with no formation, simply C/5 charge and C/2 discharge cycling at both 20°C and 40°C under low pressure are included for comparison. Surprisingly, this simple two cycle hot formation procedure has an enormous effect on the performance of the 20°C anode free cells. The cells which underwent hot formation show a much smaller fade rate, achieving 60 cycles to 80% retention compared to only 18 cycles for the 20°C control case. The magnitude of the impact of hot formation is comparable to that of applying 1200 kPa pressure. In terms of cycles to 80% retention, the hot formation 20°C cells are still slightly worse than those cycled at 40°C, however with hot formation the cells show better longer term retention after 100 cycles. When high pressure is applied to the hot formation cells, the performance is further enhanced (Figure 2a). These high pressure hot formation cells cycled at 20°C show 85% capacity retention at 100 cycles, superior to both the low and high pressure cells cycled at 40°C. The anode free cells cycled 40°C show an obvious "knee" in the capacity retention curves around cycle 80–90 where the fade rate increases. We have previously demonstrated that this knee and subsequent failure are caused by significant salt consumption from the electrolyte during cycling.21 For the hot formation cells cycled at 20°C, the long term fade rate is much more stable and there are no sharp slope changes in the capacity retention curves. At room temperature the parasitic reactions consuming electrolyte salt should be slower thereby increasing cycling life and delaying cell failure. Thus, one of the benefits of lower temperature cycling (reduced rate of parasitic reactions) can now be realized when cycling is initialized with hot formation.
Figure 2. a) Normalized discharge capacity vs. cycle number for anode-free cells cycled under low (75 kPa) and high (1200 kPa) pressure at 20°C and 40°C without hot formation as well as at 20°C with a hot (40°C) formation protocol; b) Comparison of anode-free capacity retention for cells cycled at 20°C with no formation (control), no formation and initial gas volume, single cycle hot formation with no initial gas volume, and both single cycle hot formation and initial gas volume; c) Comparison of anode-free capacity retention for cells cycled at 20°C with no formation, single cycle hot formation with initial gas volume, two-cycle hot formation with no initial gas, and two cycling hot formation with initial gas; All cells shown in plots (b) and (c) were cycled at low (75 kPa) pressure. The electrolyte in all cases was 0.6M LiDFOB 0.6M LiBF4 FEC:DEC 1:2; d) Capacity retention of anode free cells cycling with high concentration electrolyte (1.8M LiDFOB 0.4M LiBF4 FEC:DEC 1:2) at 20°C after hot formation protocol. Replicates or brother cells run for each condition are represented by open and filled symbols of the same color.
In order to better understand why hot formation is improving cell performance, it is important to consider the changes occurring during hot formation. Hot formation should allow for improved initial lithium morphology due to the slower charge rate and the higher temperature at which the lithium can be more easily constrained. Additionally, reactions occurring within the cell will be accelerated during the high temperature formation compared to lower temperature cycling. For instance, the reactions generating gas within a cell are often significantly accelerated at higher temperature. Figure S2 shows the gas generation after 50 cycles at different temperatures for anode-free cells with 0.6M LiDFOB 0.6MLiBF4 FEC:DEC 1:2 electrolyte cycled with a C/5 charge and C/2 discharge between 3.6 and 4.5 V. The gas volume was measured via the Archimedes' method described previously.24 Predictably, the gas generation increases rapidly with temperature. The LiDFOB salt is known to oxidize at high voltage with CO2 being one of the byproducts.25 GC-MS analysis of the gas produced during cycling of LiDFOB/LiBF4 anode free cells revealed CO2 as the only quantifiable gas detected to a significant degree (Figure S3). CO2 has been has been demonstrated as an effective additive for Si anodes and has been used as a SEI film forming additive in lithium-ion and lithium metal cells.26,27 Thus, some of the benefit of hot formation may be from the additional CO2 generated at the cathode and the subsequent beneficial cross-talk reactions. To explore this idea, further experiments were conducted to better quantify the impact of gas generated during formation with the resulting cycling data shown in Figure 2b.
The pink curves in Figure 2b represents results for control cells cycled (C/5 charge C/2 discharge) at 20°C. The light blue curves correspond to cells which were charged (C/5) once at 20°C, moved to a 40°C chamber and held for 8 hours to generate approximately 0.2 mL of gas, and then cycled (C/5 charge C/2 discharge) at 20°C. The production of gas after the first charge results in a noticeable but very minor improvement in capacity retention, which means gas generation alone without hot formation has minimal impact. The dark blue curves show results for cells that underwent a single C/5 hot formation cycle at 40°C and were subsequently degassed before being cycled (C/5 charge C/2 discharge) at 20°C. This single hot formation cycle, even after degassing, still has a large effect on capacity retention, more than double the number of cycles until 50% retention. This means that when isolated, hot formation has a more significant impact than gas generation. Finally, the green curves in Figure 2b corresponds to the combination of a C/5 hot formation cycle followed by an 8 hour hold to generate 0.2 mL of gas. When hot formation and gas generation are combined the best capacity retention is achieved. This is reasonable as when cycling lithium metal the SEI is constantly being partially broken and reformed during so having an additional reservoir of a helpful additive should be beneficial.
The effect of the length of the formation protocol was also investigated. The capacity retention curves for the 20°C control cells and single cycle hot formation with degas cells are reproduced in Figure 2c along with those for a two cycle (C/10 charge C/2 discharge) hot formation protocol in both the degassed and non-degassed condition. The longer two cycle hot formation protocol shows a definite benefit over the single cycle formation when comparing cell performance in the degassed condition. The slower charge may contribute to superior initial lithium morphology and the longer overall formation time could allow for more complete SEI formation. The performance improvement of the degassed two-cycle hot formation cells over the 20°C control is quite remarkable. In this condition all of the gas generated during formation is removed so it is really just the changes that occurred during those first two cycles which are having a profound impact on the subsequent cycle life, an impact that lasts beyond cycle 100. When the two cycle hot formation cells are not degassed there is an additional benefit to the cycling performance from the reservoir of CO2. It's interesting to note that the benefit that comes from not degassing the cell seems to be less substantial with the longer formation protocol compared to the single cycle formation, again suggesting that the longer process offers a more complete formation. A two cycle formation at even higher temperature (55°C) was also attempted, but this showed no benefit over the 40°C formation (Figure S4).
As mentioned, the failure of these anode free cells cycling with LiDFOB/LiBF4 dual salt electrolyte can be attributed to salt consumption.21 Therefore in order to achieve the best possible performance under current conditions we evaluated a high concentration dual salt electrolyte (1.8M LiDFOB 0.4M LiBF4 FEC:DEC 1:2) cycling in anode free cells at 20°C following a two cycle hot formation (Figure 2d). At low pressure (75 kPa) the high concentration cells maintain achieve 95–100 cycles before falling below 80% retention. With high concentration and high pressure after the two cycle hot formation protocol the anode-free cell reaches 195 cycles to 80% retention. The absolute capacity retention and difference between average charge and discharge voltages (called ΔV here) of these cells are shown in Figure S5. With a 3.6 V discharge cutoff at 20°C these cells leave approximately 30% excess lithium on the anode after the first cycle. Taking this into consideration, close to 80% retention at 200 cycles with only 30% excess lithium is among the best performance reported for lithium metal cells with limited excess. This corresponds to an average lithium metal cycling efficiency of 99.67% over 200 cycles.
Surface morphology
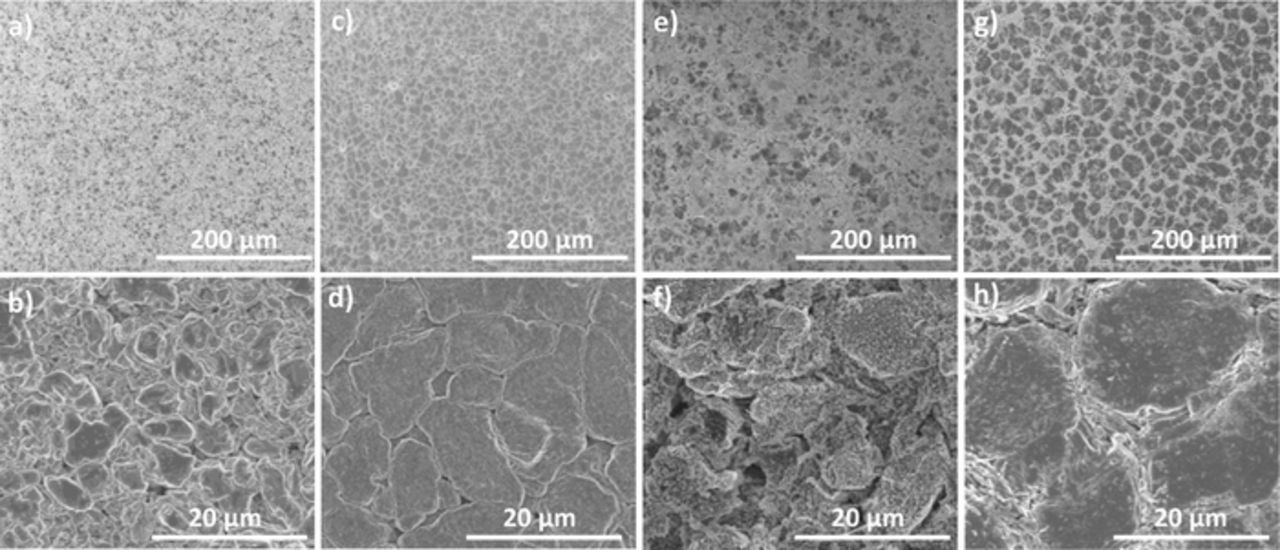
Figure 3 shows SEM images of the lithium anode at the top of charge (4.5 V) were for cells with and without hot formation. Figures 3a and 3b show the lithium anode after two cycles (C/5 Charge, C/2 Discharge) at 20°C with low (75 kPa) applied pressure. In the higher magnification image (Figure 3b) there are some smooth, nodular lithium grains 2–4 μm in diameter, but these are dispersed amongst what appears to be a more irregular shaped and porous lithium morphology. The smooth lithium grains are regions of higher contrast, while the more porous lithium appears as lighter regions. Thus, one can clearly see in the lower magnification image (Figure 3a) that these smooth high contrast regions are the minority of the sample. Conversely, the morphology of the lithium anode after two cycles of hot formation (C/10 charge; C/2 discharge at 40°C) at low (75 kPa) pressure looks much better (Figures 3c and 3d). After two cycles at the higher temperature the lithium has a smooth and flat columnar morphology with lithium grains 5–10 μm in diameter. Not only are the lithium grains larger at higher temperature, but they are also packed much more tightly leading to much less porosity. The lower magnification image (Figure 3c) shows that this columnar morphology is uniform over a larger area. It certainly appears that two cycles at higher temperature leads to superior lithium morphology and so the lithium quality after hot formation is far superior to that demonstrated after two conventional 20°C cycles.
Figure 3. Low (a,c,e,f) and high (b,d,f,g) magnification SEM images of lithium anodes at the top of charge (4.5V) from NMC 532 || Cu pouch cells cycled with 0.6M LiDFOB 0.6M LiBF4 FEC:DEC 1:2 electrolyte after two cycles at 20°C (a and b); after two hot formation cycles at 40°C (c and d); after 20 cycles at 20°C with no hot formation (e and f); after 20 cycles at 20°C following a two cycle hot formation protocol (g and h).
While the hot formation provides a better starting point, it's important to determine if this superior morphology can be retained after subsequent lower temperature cycling. Figures 3e and 3f show the lithium anode after 20 cycles (C/5 charge C/2 discharge) at 20°C in a cell that did not have hot formation. The higher magnification image (Figure 3f) displays many irregular geometries with no smooth flat grains leading to an overall porous morphology. The lower magnification image (Figure 3e) shows that the low contrast regions of poor morphology represent the majority of the sample. This poor quality high surface area lithium explains the fast capacity fade shown by the cells cycled at 20°C without formation.
Figures 3g and 3h show the lithium anode after 20 cycles (C/5 charge C/2 discharge) at 20°C for a cell that had hot formation. The difference between the morphology with and without hot formation is pretty dramatic. The low magnification image (Figure 3g) shows that the darker high contrast regions of flat lithium columns now represent the majority of the sample. At higher magnification (Figure 3h) these regions appear as large flat lithium grains >20 μm in diameter, quite different from the irregular geometries and porous structure shown after 20 cycles without formation (Figures 3e and 3f). Even with hot formation there seems to be a porous lithium morphology growing between the large grains, however at this point it represents a small portion of the sample. The SEM analysis confirms what the cycling data suggested; the benefits established during the two hot formation cycles are carried forward and have a substantial impact the quality of lithium plated on subsequent cycles and in turn on the cycling stability of the anode free cells.
Conclusions
Temperature strongly influences the cycling stability of anode-free lithium metal pouch cells. When the cycling temperature was reduced from 40°C to 20°C, the capacity retention of NMC 532 || Cu pouch cells under low applied pressure (75 kPa) decreased dramatically. Increasing the applied pressure to 1200 kPa helped to significantly improve low temperature cycling performance, which aligns with recent reports asserting that more force is required to deform lithium metal at lower temperature. Therefore, it seems that lower cycling temperatures may require higher pressure to properly constrain the lithium metal anode.
Understanding the benefits of higher temperature cycling, a "hot formation" protocol was proposed involving two initial high temperature (40°C) cycles before extended lower temperature cycling. These two initial cycles had a surprising effect on the capacity retention of anode-free cells cycling with LiDFOB/LiBF4 dual salt electrolyte at low pressure; the number of cycles to 80% retention increased from only 18 without hot formation to close to 60 with hot formation. When the pressure was increased to 1200 kPa these hot formation cells achieved 85% retention at 100 cycles. The hot formation protocol was also combined with a high concentration dual salt electrolyte to further improve cycling stability enabling an average CE of 99.67%over 200 cycles; among the highest values demonstrated for lithium metal cells with limited lithium excess. A trade-off for improved capacity retention is that at 20°C the initial cell capacity is 210 mAh, compared to 250 mAh at 40°C due to increased cell impedance at lower temperature.
The benefits of hot formation could be partially attributed to increased gas (CO2) generation during hot formation which acted as a beneficial additive. However, even with cell degassing, the benefits of hot formation were maintained throughout cycling. Lithium morphology after the first two hot formation cycles was far superior to that shown after two initial low temperature cycles. This morphology improvement with hot formation was maintained even after additional low temperature cycles. Thus, the benefits of a short two cycles of a hot formation protocol can be carried forward to improve the low temperature cycling performance of anode-free lithium metal cells.
ORCID
A. J. Louli 0000-0001-9819-1324
J. R. Dahn 0000-0002-6997-2436