Abstract
The products formed by the chemical extraction of lithium from LiMn2-xMxO4 (M = Cr, Fe, Co, and Ni) with aqueous sulfuric acid have been structurally and chemically characterized. The amount of lithium extracted is found to be proportional to the initial Mn3+ content, confirming the reaction mechanism, LiMn3+Mn4+O4 → 0.75 Mn2O4 + 0.5 Li2O + 0.5 MnO. Thus, no lithium is extracted from LiMn1.54+Ni0.52+O4 as there is no Mn3+, while complete delithiation occurs in LiMn3+Mn4+O4 to give Mn2O4. The amount of lithium extracted from the other samples is in good agreement with that calculated from the Mn3+ content. The XRD data confirm the maintenance of the spinel structure for the delithiated samples, while the redox titration confirmed the maintenance of oxygen content at 4.0, indicating that no protons were inserted into the lattice during delithiation. The findings could provide more insight on the problem of metal dissolution from cathode materials in lithium-ion batteries.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Lithium-ion batteries have become an integral part of modern society as they power most of the consumer electronic devices due to their high energy density; they are also being intensively pursued for electric vehicles (EV) and plug-in hybrid electric vehicles (PHEV).1 Lithium-insertion compounds based on layered, spinel, and olivine structures, e.g., LiCoO2, LiMn2O4, LiFePO4, as well as their solid solutions with other metal ions, have become the dominant cathode materials for lithium-ion batteries.2 One of the major issues with lithium-ion batteries is the dissolution of transition-metal ions from the cathode into the electrolyte, especially at higher operating voltages and under conditions of over charge.3–6 The dissolved metal ions migrate into the graphite anode, get reduced, plate on the anode, and lead to performance degradation during cycling.7 The amount of metal ions dissolved from the cathode is generally determined by analyzing the transition-metal ion content on the cycled anode or by soaking the cathode powder in electrolyte for a certain amount of time and analyzing the transition-metal-ion content in the electrolyte. It is widely accepted that manganese ions dissolve more than other ions like cobalt or nickel and, therefore, the manganese ions play a larger role in poisoning the graphite anode.
The manganese dissolution in lithium-ion cells is believed to occur due to a disproportionation of Mn3+ into Mn4+ and Mn2+ in the presence of trace amounts of protons originating from ppm levels of H2O, which generates HF by reacting with the LiPF6 salt in the electrolyte. This is supported by an elegant experiment carried out by Hunter in 1981.8 He showed that stirring the spinel LiMn2O4 powder with dilute sulfuric acid resulted in an extraction of lithium from LiMn2O4 due to the disproportionation of Mn3+ into Mn4+ and Mn2+ ions to give MnO2 as shown in Reaction 1:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/162/3/A426/revision1/jes_162_3_A426eqn1.jpg)
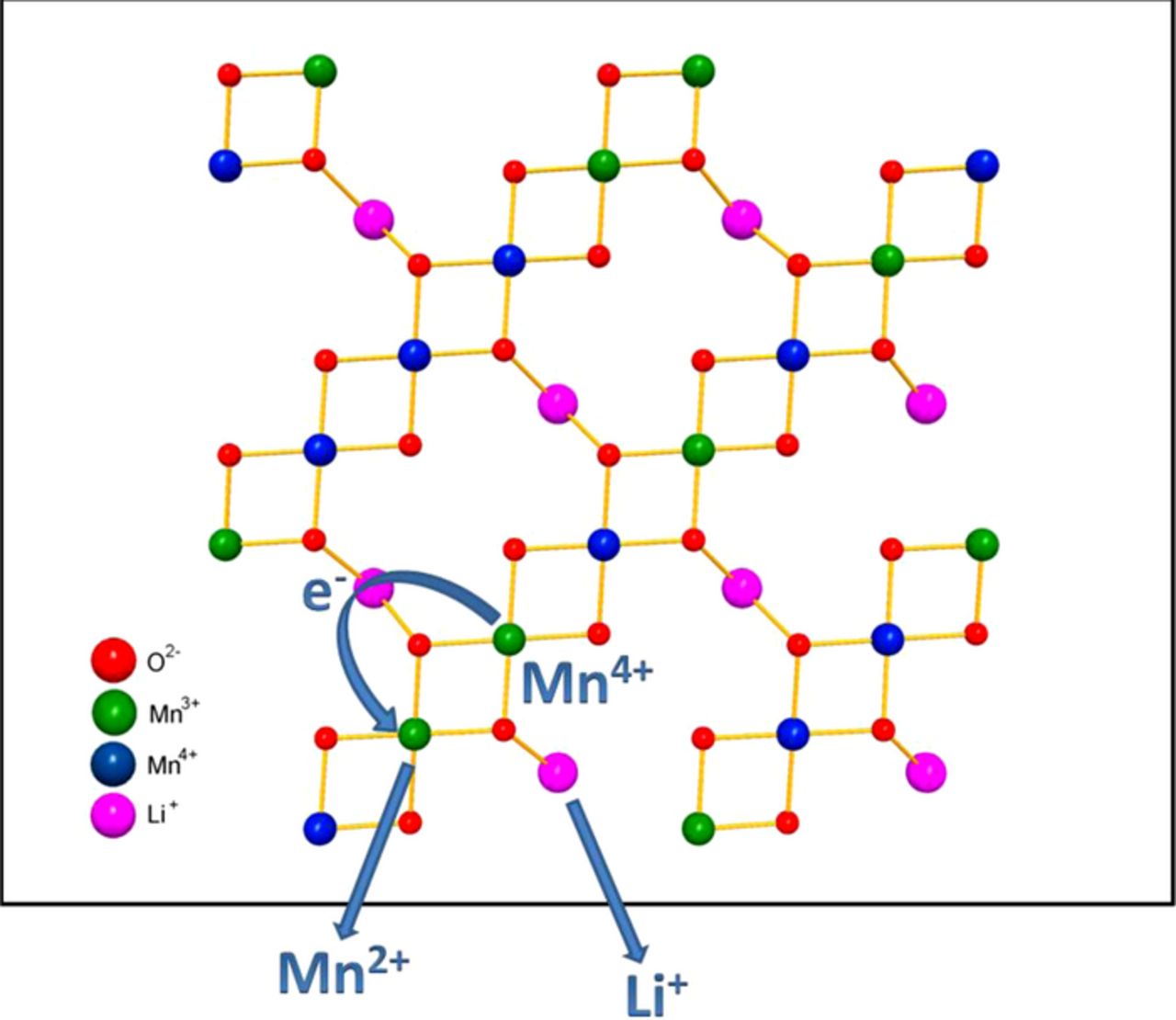
While both Li2O and MnO go into solution as Li2SO4 and MnSO4 by reaction with sulfuric acid, Mn2O4 (or MnO2) remains in the solid with the maintenance of the spinel framework structure in which the 8a tetrahedral sites are completely empty. This spinel form of MnO2 is designated commonly as λ-MnO2. The lithium extraction process in acid medium from spinel LiMn2O4 following Reaction 1 is pictorially depicted in Figure 1.
Figure 1. Atomic model depicting the mechanism of lithium extraction from spinel LiMn2O4 with acid as proposed by Hunter.8
Following the early work by Hunter, several studies have focused on the extraction of lithium from LiMO2 (M = Mn, Co, and Ni) layered oxides.9–12 However, the delithiation of layered oxides with dilute sulfuric acid is often complicated by an exchange of Li+ ions in the lattice by H+ ions. Protons are inserted into the layered oxides even when lithium extraction is carried out in a nonaqueous medium such as acetonitrile with oxidizing agents like NO2BF413,14 and chlorine.12 In fact, the ability of layered oxides to readily accommodate protons into the lithium layer seems to adventitiously improve the cyclability of spinel LiMn2O4 when they are employed together in a cathode because the layered oxide can trap the trace amount of protons present in the electrolyte and suppress manganese dissolution from the spinel phase.15 For example, composite cathodes consisting of spinel LiMn2O4 and layered Li[Mn,Ni,Co]O2 (∼2:1 ratio) are used effectively in the Chevy Volt and Nissan Leaf to overcome the common challenge with spinel LiMn2O4 cathodes. Interestingly, the extraction of lithium from spinel LiMn2O4 with the oxidizing agent NO2BF4 does not seem to be accompanied by an exchange of Li+ ions with protons since the oxidation state of Mn (determined by a redox titration) increases proportionately with the degree of lithium extraction from LiMn2O4.16 However, there have been some reports on the exchange of Li+ ions by protons in spinel oxides,17–19 but further clarification is necessary. It seems that such an exchange occurs only when the lithium content is >1.0 or with spinel oxides prepared at low temperatures that have cation defects.
The layered and spinel oxide cathodes are based mostly on Mn, Co, and Ni; however, the substitutions of small amounts of Cr and Fe have shown improvement in performance, particularly with the high voltage spinel LiMn1.5Mn0.5O4.20–25 In order to develop a better understanding of the mechanisms that lead to capacity fade, a comprehensive understanding of which ions among Cr, Mn, Fe, Co, and Ni tend to disproportionate and which ions do not in the presence of acid needs to be developed. Since the delithiation reactions of layered oxides with acid are complicated by the exchange of Li+ ions by H+ ions, it is difficult to establish the disproportionation reaction phenomenon in layered oxides. Spinel oxides synthesized at high temperatures with a lithium content of 1.0 are the best materials to establish this phenomenon as they do not seem to incorporate protons,16 but spinel oxides like LiCo2O4 and LiNi2O4 that would contain Co3+ and Ni3+ ions are not known as pure well-developed spinel samples. Accordingly, we present here a systematic investigation of the delithiation with acid of four series of spinel oxides, LiMn2-xNixO4 (0 ≤ x ≤ 0.5) and LiMn2-xMxO4 (0 ≤ x ≤ 1.0) (M = Cr, Fe, and Co). The products formed before and after delithiation with acid are characterized by X-ray diffraction (XRD), lithium content analysis, and oxygen content analysis to establish (i) the delithiation mechanism, (ii) whether or not Cr3+, Fe3+, Co3+, and Ni2+ ions also disproportionate similar to Mn3+, and (iii) whether or not protons are inserted into the spinel lattice due to an exchange of Li+ ions by H+ ions.
Experimental
Synthesis
The spinel LiMn2-xMxO4 (M = Cr, Fe, Co, and Ni) samples were synthesized by a solution-based method employing ethylenediaminetetraacetic acid (EDTA) and citric acid as chelating agents, analogous to that reported previously for layered oxides.26 Required amounts of lithium, manganese, nickel, iron, and cobalt acetate and chromium nitrate were dissolved in water, while a separate aqueous solution consisting of EDTA, citric acid, and ammonium hydroxide was also prepared. The metal acetate solution was then added dropwise into the EDTA/citric acid/NH4OH solution, while maintaining a metal ions : EDTA : citric acid molar ratio of 1:1:1.5. The transparent solution formed was stirred for 12 h at ∼120°C to obtain a viscous gel, which was then heated at ∼450°C for ∼6 h until the organics mostly decomposed. The resulting powder was then calcined at 800°C for 24 h. The LiMn1.5Ni0.5O4 sample was annealed at 700°C for 48 h to eliminate most of the rocksalt impurity, which also increased the degree of cation ordering between Mn4+ and Ni2+ ions. The delithiation procedure consisted of suspending 250 mg of the spinel oxide in 25 mL of 0. 35 N H2SO4 and stirring it for 24 h at room temperature, followed by filtering with a sintered glass filter, washing thoroughly with de-ionized water to remove the acid and other soluble products completely, and drying in an air oven at 100°C overnight. It should be noted that much higher solution acidity than would be experienced inside an actual lithium-ion cell is used here in order to speed up the delithiation process to occur at a reasonable time scale and assess the products and understand the mechanism.
Characterization
The samples were characterized by XRD with a Rigaku Ultima IV diffractometer with Cu K-α radiation in the 2θ range of 10–80° with a 0.03° step size. Lattice parameters were calculated using the Reitveld refinement program in Rigaku's PDXL software. Lithium and transition-metal contents in the sample were determined with a Varian 715-ES inductively coupled plasma–optical emission spectroscopy (ICP-OES) analyzer. The compositions of the initial samples were found to be close to the nominal compositions, with a Li content of 1.00 ± 0.03. The oxygen content in the samples was determined by the iodimetric redox titration.27 A known amount of the sample was dissolved in a mixture of potassium iodide and a 3.5 N HCl solution. The liberated iodine was titrated against a 0.03 N sodium thiosulfate solution using a starch solution as an indicator. Based on the amount of sodium thiosulfate consumed, the oxygen content in the sample was calculated employing the charge neutrality principle.
Results and Discussion
As pointed out in the introduction, the delithiation of spinel oxides with acid has been proposed to occur by a disproportionation of Mn3+ ions into Mn2+ and Mn4+ ions that is accompanied by the loss of soluble Li2O and MnO into the solution while Mn4+ remains in the solid (Reaction 1).8 Using this mechanism and assuming only Mn3+ undergoes disproportionation, the expected (calculated) compositions after delithiation with acid are given in Table I for the LiMn2-xNixO4 (0 ≤ x ≤ 0.5) and LiMn2-xMxO4 (0 ≤ x ≤ 1.0) (M = Cr, Fe, and Co) series. The oxidation states of the ions in the initial samples are indicated in column 2, the species dissolving into the solution are given in column 3, and the calculated compositions of the solid remaining after delithiation are given in column 4 of Table I. The compositions in column 4 are then normalized to obtain an oxygen content of 4 to be consistent with the spinel formula and are given in column 5. As the transition-metal dopant content in the initial samples increases, the amount of Mn3+ content decreases (column 2 in Table I).
Table I. Initial compositions and calculated compositions after acid delithiation based on the disproportionation of Mn3+ as shown in Reaction 1.
| Initial compositiona | Oxidation state of metal ions in the initial composition | Calculated amounts of species dissolved into the solution | Calculated solid composition after delithiation | Calculated normalized solid composition after delithiation |
|---|---|---|---|---|
| LiMn2O4 | Li+Mn3+Mn4+O42− | 0.5 Li2O + 0.5 MnO | Mn1.54+O3.0 | Mn2O4 |
| LiMn1.75Ni0.25O4 | Li+Mn0.53+Mn1.254+Ni0.252+O42− | 0.25 Li2O + 0.25 MnO | Li0.5Mn1.54+Ni0.252+O3.5 | Li0.57Mn1.71Ni0.29O4 |
| LiMn1.5Ni0.5O4 | Li+Mn1.54+Ni0.52+O42− | No disproportionation | LiMn1.54+Ni0.52+O4 | LiMn1.5Ni0.5O4 |
| LiMn1.75M0.25O4 | Li+Mn0.753+Mn4+M0.253+O42− | 0.375 Li2O + 0.375 MnO | Li0.25Mn1.3754+M0.253+O3.25 | Li0.31Mn1.69M0.31O4 |
| LiMn1.5M0.5O4 | Li+Mn0.53+Mn4+M0.53+O42− | 0.25 Li2O + 0.25 MnO | Li0.5Mn1.254+M0.53+O3.5 | Li0.57Mn1.43M0.57O4 |
| LiMn1.25M0.75O4 | Li+Mn0.253+Mn4+M0.753+O42− | 0.125 Li2O + 0.125 MnO | Li0.75Mn1.1254+M0.753+O3.75 | Li0.8Mn1.2M0.8O4 |
| LiMnMO4 | Li+Mn4+M3+O42− | No disproportionation | LiMn4+M3+O4 | LiMnMO4 |
Figures 2 through 5 compare the XRD patterns of the LiMn2-xNixO4 (0 ≤ x ≤ 0.5) samples, the LiMn2-xCoxO4 (0 ≤ x ≤ 1.0) samples, the LiMn2-xCrxO4 (0 ≤ x ≤ 1.0) samples, and the LiMn2-xFexO4 (0 ≤ x ≤ 1.0) samples, respectively, before and after delithiation. Their lattice parameters can be seen in Table II. All of the reflections in the samples before and after delithiation could be indexed based on the Fd-3m cubic spinel structure, indicating the absence of impurity phases, except for a few samples. A trace amount of Ni1-xLixO impurity can be found in the x = 0.5 sample in the Ni series. In the case of LiMnCoO4, a small amount of a Co-rich layered oxide phase is present. In the Fe series, when x ≥ 0.75, there are several additional peaks on the lower 2θ side of the existing peaks, which are most likely from an Fe-rich spinel impurity.28,29 The initial LiMn2-xCoxO4 samples in the Co series exhibit increased peak broadening with increasing Co content. The Co and Mn ions do not order in the spinel structure, so increasing the Co content increases the structural disorder and leads to peak broadening. However, the trend reverses upon going from the x = 0.75 sample to the x = 1.0 sample, as the 1:1 ratio of Mn:Co may provide a better structural order and crystallinity due to the lack of Mn3+ ions. This behavior was also seen by Amarillo et al.30 Although another study has reported Cr ions in the 8a tetrahedral sites depending on the composition and synthesis method,31 this was not found to be the case in our study as the (111) peaks at ∼19° did not show a decrease in intensity and the (220) peaks at ∼31° did not exhibit an increase in intensity as would occur if an appreciable amount of Cr were in the tetrahedral sites.29,32
Table II. Compositions, lattice parameters, and oxygen content values before and after delithiation with acid.
| Initial samples | Samples obtained after delithiation | ||||||
|---|---|---|---|---|---|---|---|
| Composition | Lattice parameter (Ǻ) | Oxygen contenta | Calculated compositionb | Experimentally observed composition | Lattice parameter (Ǻ) | Change in lattice parameter (Ǻ) | Oxygen contenta |
| LiMn2O4 | 8.24140(6) | 4.01 | Mn2O4 | Li0.07Mn2.00O4 | 8.03665(5) | −0.20475 | 3.98 |
| LiMn1.75Ni0.25O4 | 8.19559(3) | 3.98 | Li0.57Mn1.71Ni0.29O4 | Li0.56Mn1.71Ni0.24O4 | 8.12134(3) | −0.07425 | 3.98 |
| LiMn1.5Ni0.5O4 | 8.17194(2) | —— | LiMn1.5Ni0.5O4 | Li1.01Mn1.5Ni0.50O4 | 8.17122(2) | −0.00072 | —– |
| LiMn1.75Co0.25O4 | 8.18126(3) | 4.02 | Li0.31Mn1.69Co0.31O4 | Li0.31Mn1.69Co0.26O4 | 8.06331(2) | −0.11795 | 4.01 |
| LiMn1.5Co0.5O4 | 8.12514(2) | 3.99 | Li0.57Mn1.43Co0.57O4 | Li0.58Mn1.43Co0.52O4 | 8.05857(4) | −0.06657 | 4.02 |
| LiMn1.25Co0.75O4 | 8.08457(4) | 3.98 | Li0.8Mn1.2Co0.8O4 | Li0.75Mn1.2Co0.76O4 | 8.05642(6) | −0.02815 | 3.97 |
| LiMnCoO4 | 8.05679(3) | —– | LiMnCoO4 | Li0.91MnCo1.00O4 | 8.04968(8) | −0.00711 | —– |
| LiMn1.75Cr0.25O4 | 8.22183(3) | 3.97 | Li0.31Mn1.69Cr0.31O4 | Li0.28Mn1.69Cr0.26O4 | 8.10656(5) | −0.11527 | 4.02 |
| LiMn1.5Cr0.5O4 | 8.20536(4) | 4.03 | Li0.57Mn1.43Cr0.57O4 | Li0.54Mn1.43Cr0.51O4 | 8.14018(3) | −0.06518 | 4.03 |
| LiMn1.25Cr0.75O4 | 8.19352(4) | —– | Li0.8Mn1.2Cr0.8O4 | Li0.81Mn1.2Cr0.77O4 | 8.17403(4) | −0.01949 | —– |
| LiMnCrO4 | 8.18917(4) | —– | LiMnCrO4 | Li1.01MnCr0.99O4 | 8.18639(4) | −0.00278 | —– |
| LiMn1.75Fe0.25O4 | 8.23916(4) | 4.01 | Li0.31Mn1.69Fe0.31O4 | Li0.30Mn1.69Fe0.29O4 | 8.12548(4) | −0.11368 | 3.97 |
| LiMn1.5Fe0.5O4 | 8.25261(4) | 4.03 | Li0.57Mn1.43Fe0.57O4 | Li0.59Mn1.43Fe0.49O4 | 8.17862(5) | −0.07399 | 4.02 |
| LiMn1.25Fe0.75O4 | 8.32608(4) | —– | Li0.8Mn1.2Fe0.8O4 | Li0.62Mn1.2Fe0.74O4 | 8.21365(8) | −0.11243 | —– |
| LiMnFeO4 | 8.24981(3) | —– | LiMnFeO4 | Li0.85MnFe1.03O4 | 8.30227(9) | 0.05246 | —– |
aThe oxygen content values for some compositions could not be determined as the samples could not be fully dissolved. bThe calculated compositions were obtained as illustrated in Table I.
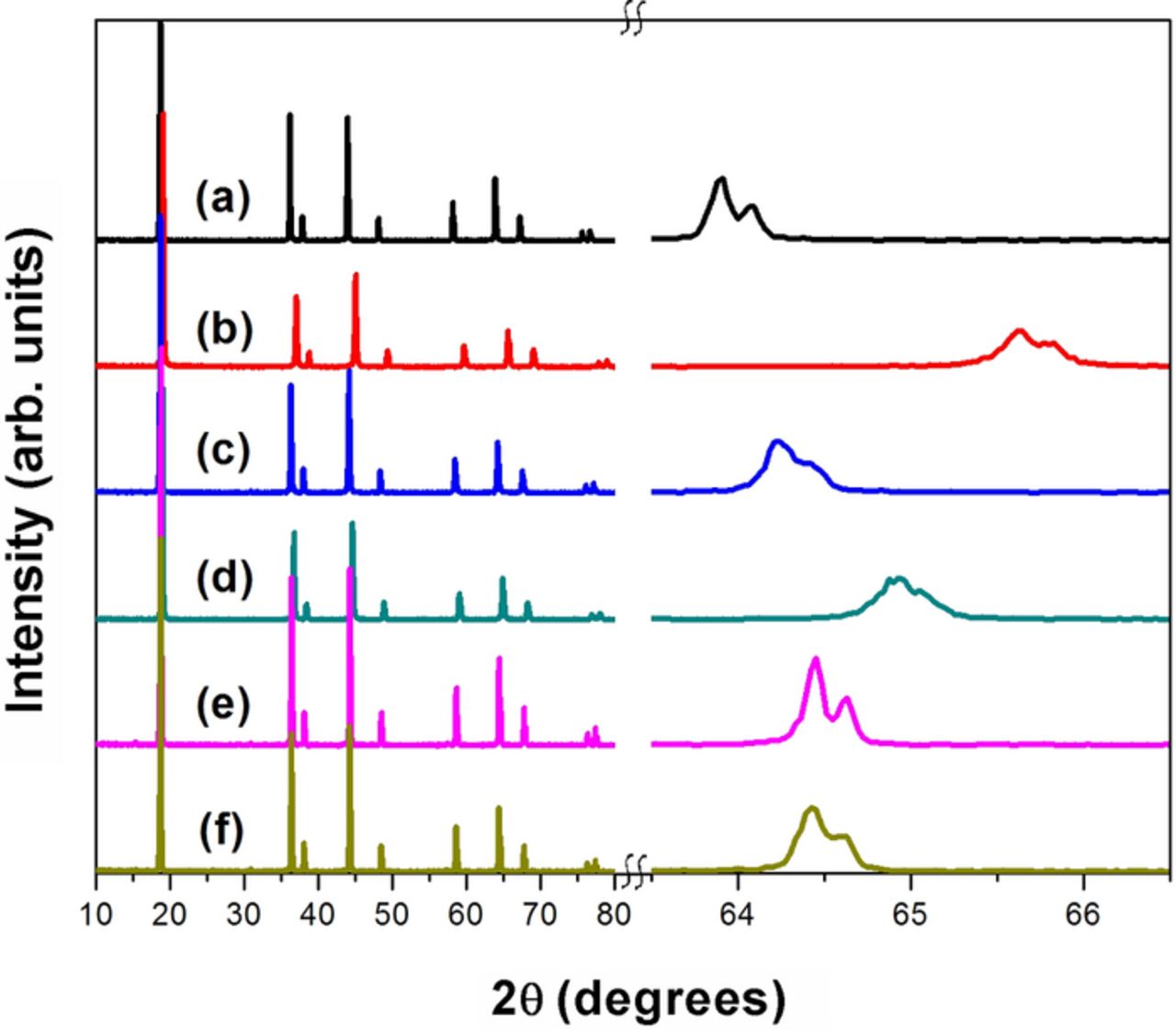
Figure 2. XRD patterns of LiMn2-xNixO4 series before and after delithiation with acid. The expanded region over a small 2θ range on the right reveals the shifts in the peaks to higher angles upon delithiation. (a) x = 0 before delithiation, (b) x = 0 after delithiation, (c) x = 0.25 before delithiation, (d) x = 0.25 after delithiation, (e) x = 0.5 before delithiation, and (f ) x = 0.5 after delithiation.
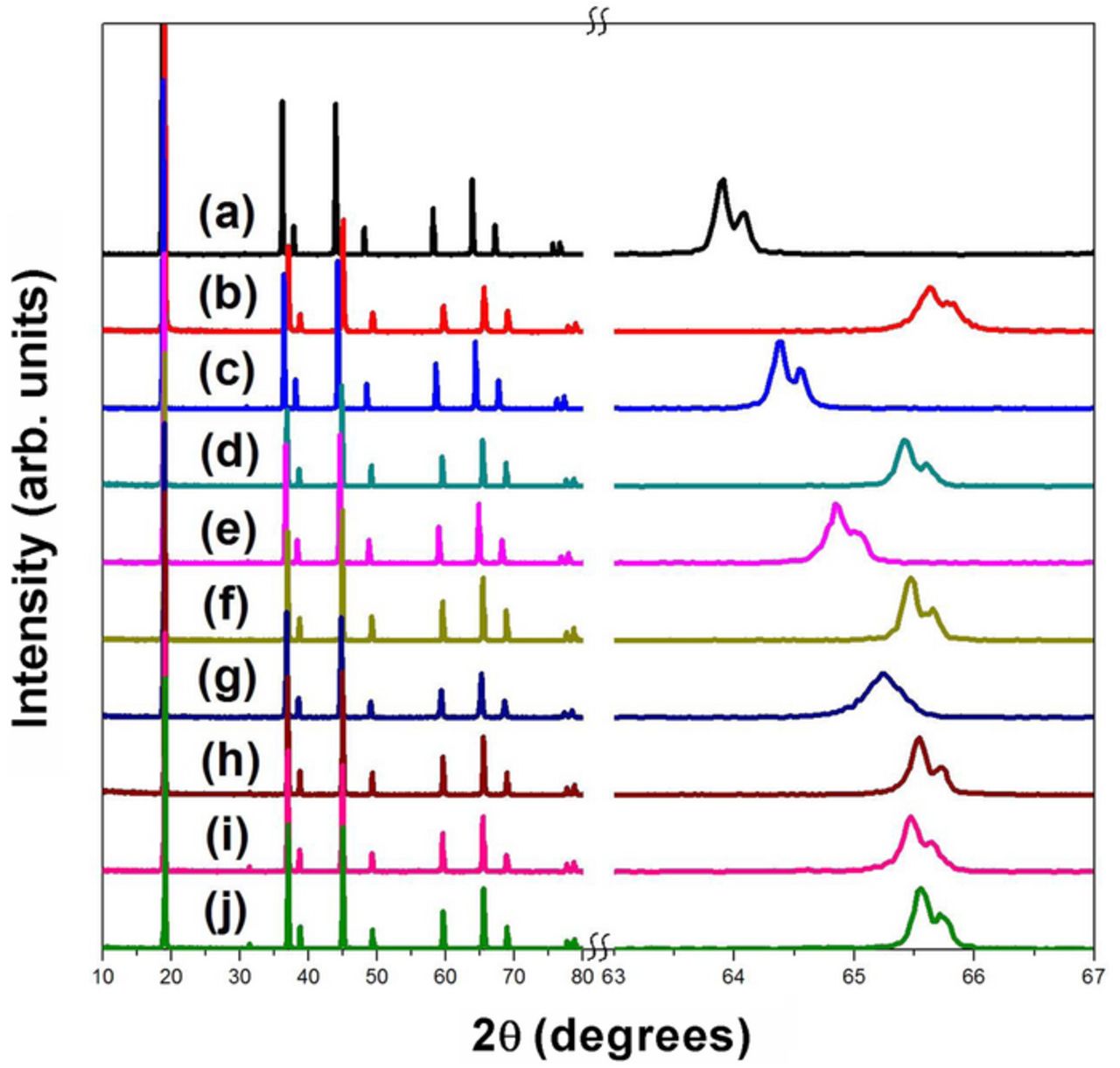
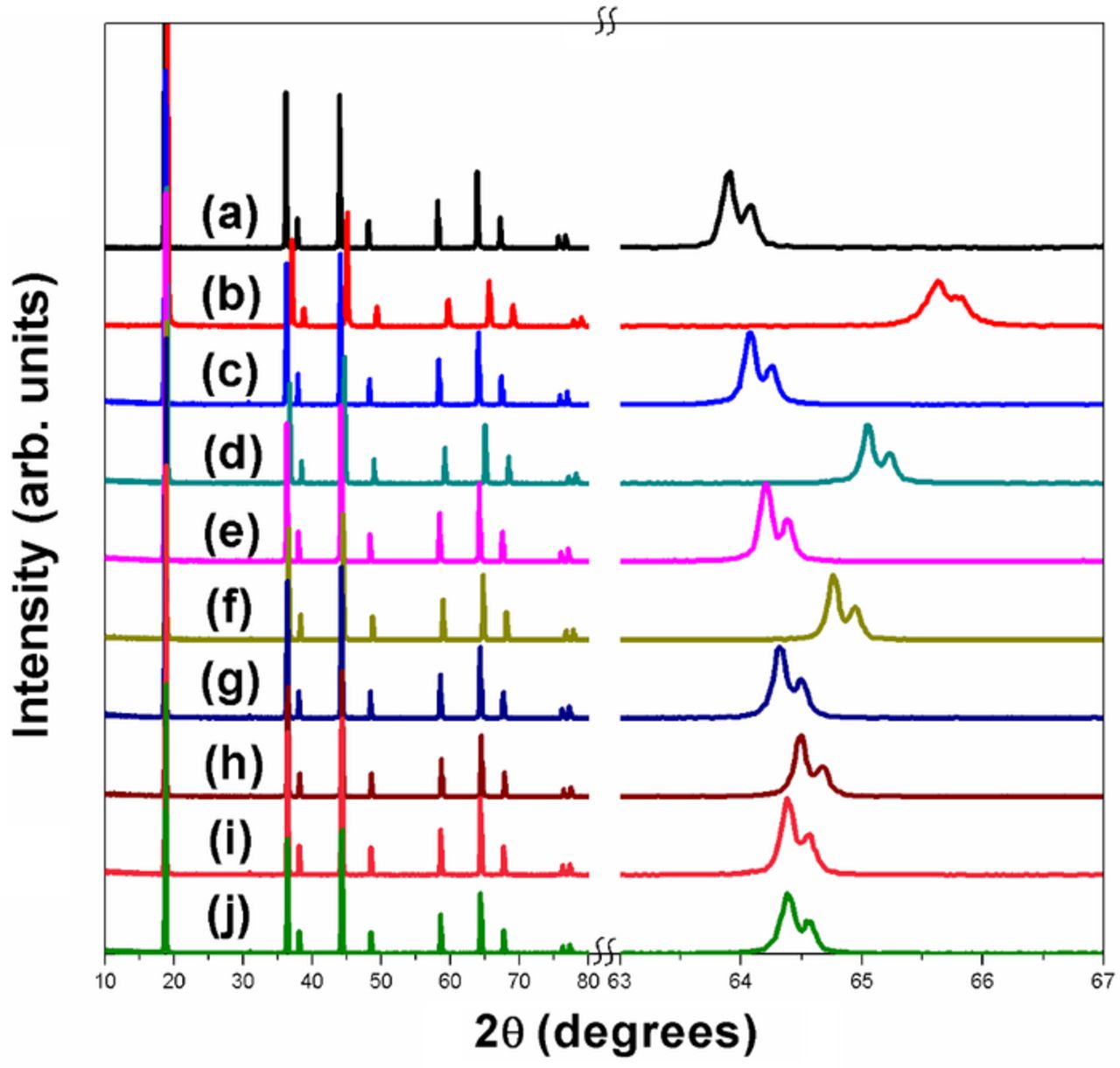
Figure 3. XRD patterns of LiMn2-xCoxO4 series before and after delithiation with acid. The expanded region over a small 2θ range on the right reveals the shifts in the peaks to higher angles upon delithiation. (a) x = 0 before delithiation, (b) x = 0 after delithiation, (c) x = 0.25 before delithiation, (d) x = 0.25 after delithiation, (e) x = 0.5 before delithiation, (f ) x = 0.5 after delithiation, (g) x = 0.75 before delithiation, (h) x = 0.75 after delithiation, (i) x = 1 before delithiation, and (j) x = 1 after delithiation.
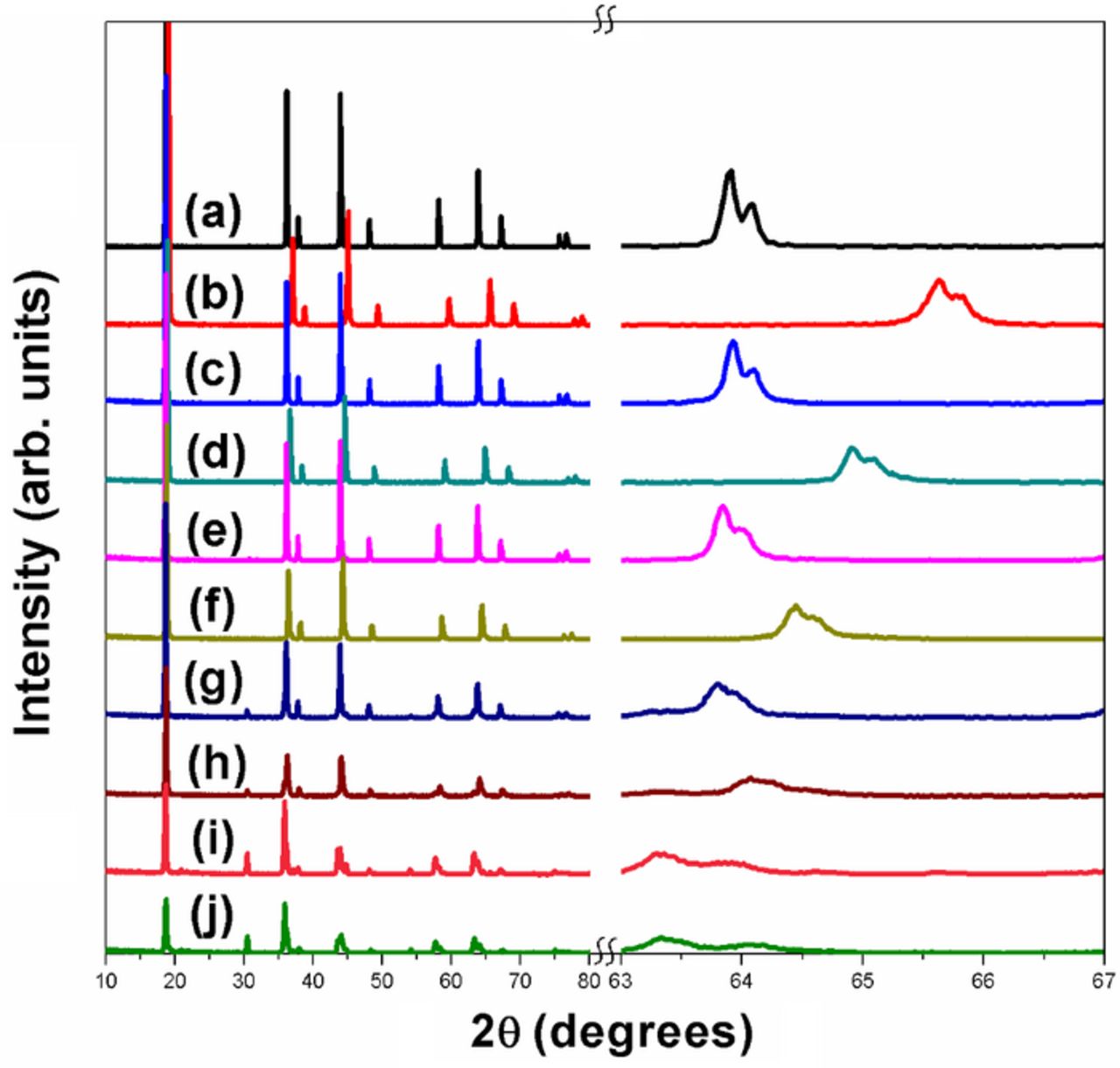
Figure 5. XRD patterns of LiMn2-xFexO4 series before and after delithiation with acid. The expanded region over a small 2θ range on the right reveals the shifts in the peaks to higher angles upon delithiation. (a) x = 0 before delithiation, (b) x = 0 after delithiation, (c) x = 0.25 before delithiation, (d) x = 0.25 after delithiation, (e) x = 0.5 before delithiation, (f ) x = 0.5 after delithiation, (g) x = 0.75 before delithiation, (h) x = 0.75 after delithiation, (i) x = 1 before delithiation, and (j) x = 1 after delithiation.
The peaks in the as-prepared Co and Cr samples shift to higher 2θ values as the dopant content increases due to the substitution of smaller Co3+ ions (0.61 Ǻ) and Cr3+ ions (0.615 Ǻ) for the larger Mn3+ ions (0.645 Ǻ). The peaks in the as-prepared Ni samples also shift to higher 2θ values as the Ni content increases since the substitution of Ni for Mn creates a Ni2+ ion (0.69 Ǻ) and a Mn4+ ion (0.53 Ǻ) at the expense of two Mn3+ ions (0.645 Ǻ), so the net effect is a decrease in lattice parameter. However, the peaks in the as-prepared Fe samples have relatively constant locations with increasing Fe content since Fe3+ ions (0.645 Ǻ) and Mn3+ ions (0.645 Ǻ) have identical size. Except for the materials where no delithiation occurs, the peaks in the delithiated samples all shift to higher 2θ values and the lattice parameter values given in Table II decrease due to the oxidation of the larger Mn3+ ions to smaller Mn4+ ions. As expected, the shift to higher 2θ values and the change in lattice parameters after delithiation decrease with increasing dopant content due to the decrease in Mn3+ content in the initial samples and amount of the disproportionation Reaction 1. For the highest-doped sample in each series, which contains little or no Mn3+, the reflections should not shift and the lattice parameters should not change after the delithiation treatment with the acid. This is true for the Ni and Cr series. However, LiMnCoO4, which may contain a small amount of Mn3+ due to the Co-rich layered oxide impurity, shows a very small change in lattice parameter after the acid-delithiation treatment. The difficulty in keeping all Mn as Mn4+ in LiMnCoO4 during the high-temperature synthesis may lead to the small amount of Mn3+, which would be accompanied by the presence of a trace amount of layered oxide impurity phase or oxygen deficiency. Similarly, LiMnFeO4, which also contains an impurity phase, exhibits a slight peak shift and lattice parameter change upon delithiation.
Table II gives the experimentally obtained compositions based on the Li, Cr, Mn, Fe, Co, and Ni contents determined by ICP-OES analysis for the LiMn2-xNixO4 (0 ≤ x ≤ 0.5) and LiMn2-xMxO4 (0 ≤ x ≤ 1.0) (M = Co, Cr, and Fe) series. As seen in Table II, the experimentally determined lithium contents in the samples match well with the values calculated based on the disproportionation reaction of Mn3+ (Reaction 1). The experimental compositions of the transition-metal dopants in column 5 are all slightly lower than the expected values in column 4, except the samples where no delithiation occurs. To investigate this issue, the dilute sulfuric acid solvent was also tested using the ICP, and it was found that 0.03–0.04 moles of Cr, Fe, Co, and Ni were dissolved in addition to the expected amounts of Li and Mn. This explains the discrepancy between the actual and expected transition-metal contents found in Table II. This can be understood by the fact that as Li2O and MnO are dissolved from the solid, the transition-metal dopants left on the surface may slightly dissolve into the sulfuric acid medium during the extended reaction time. This dissolution, however, does not cause any additional delithiation.
There are a few other discrepancies in Table II as well. Although no lithium is expected to be extracted from LiMnCoO4, the small amount of lithium extracted from it is due to the following. As pointed out by Andriiko et al.,33 LiMnCoO4 lies just past a phase boundary (LiMn1.1Co0.9O4 was the homogenous limit) and two phases are formed: one is the desired spinel phase, while the other is a Co-rich layered oxide phase. From our XRD pattern, we can see that our LiMnCoO4 sample shows a small amount of layered oxide impurity phase. Because Co is preferentially present in the layered oxide impurity phase, the spinel phase is slightly richer in Mn and deficient in Co compared to the nominal composition of LiMnCoO4. This results in a small amount of Mn3+ in the spinel phase and a consequent extraction of a small amount of lithium from the spinel phase. A similar reason also causes the delithiated x = 0.75 and 1.00 samples in the Fe series to contain much less Li than expected. As mentioned above in the XRD discussion, those two samples have significant amounts of an Fe-rich spinel impurity phase: one closer to Fe3O4 and the other closer to LiMn2O4. The LiMn2O4-like phase would experience delithiation due to its Mn3+ content.
These data presented in this investigation with four series of samples clearly establish that acid delithiation of spinel oxides occurs by a disproportionation of Mn3+ into Mn4+ and Mn2+ that is accompanied by a leaching out of corresponding amounts of Li2O and MnO into solution according to Reaction 1 proposed by Hunter.8 The data also establish that in spinel solid solutions consisting of Li, Mn, and either Cr, Fe, Co, or Ni, the amount of lithium extracted with acid is proportional to the Mn3+ content in the initial sample. Thus, the amount of lithium extracted with acid could be used to reveal quantitatively the Mn3+ content in such complex spinel oxides.
The experimental composition data presented in Table II reveal that M3+ = Cr3+, Fe3+, and Co3+ ions in spinel oxides do not disproportionate into M4+ and M2+. For example, if Co3+ ions also disproportionate similar to Mn3+ ions, then one would expect to obtain spinel-like λ-MnCoO4, but that is not the case. The same statements can be made for Cr3+ and Fe3+ ions as well. However, lithium extraction with acid has been reported in the literature with layered oxides such as LiCoO2 and LiNi1-xCoxO2.9–12 This could be largely related to the ion exchange of Li+ ions by H+ ions in acid. However, such ion exchange of Li+ ions by H+ ions does not occur in these spinel oxides as illustrated by the compositional analysis data in Table II, which shows final compositions very close to the expected values. If such an ion exchange occurred, then the calculated and observed lithium contents and compositions in Table II would not match with each other. For example, if an exchange of Li+ ions by H+ ions occurs in acid, then the LiMn1.5Ni0.5O4 composition would not remain identical (Table II) before and after treating with the acid. Because ICP analysis cannot provide compositional data for H and O, one could argue that protonation may be occurring with a corresponding increase in oxygen content, but the experimentally determined oxygen contents of 4.0 ± 0.03 for the initial materials and delithiated samples further confirm that no ion exchange has occurred during the acid delithiation process of these spinel oxides. It is possible that the protons could not be accommodated or stabilized in the 8a tetrahedral sites of the spinel lattice, while they may be readily accommodated or stabilized in the octahedral sites of the lithium layer in layered oxides.
Furthermore, the Ni2+/3+ redox energy is close to that of Mn3+/4+ energy in oxides, so one may anticipate the following equilibrium under some conditions:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/162/3/A426/revision1/jes_162_3_A426eqn2.jpg)
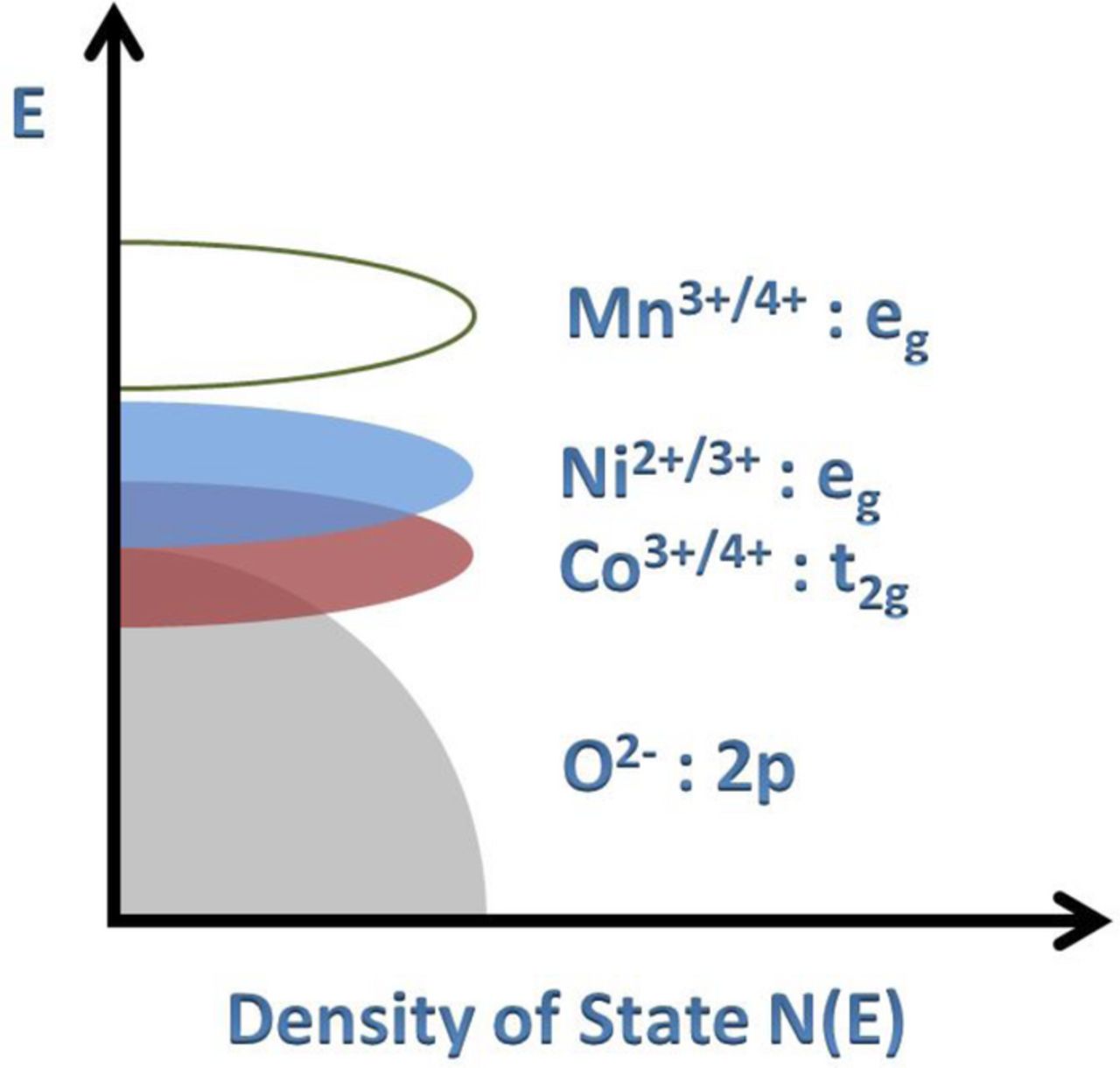
The fact that no lithium could be extracted from LiMn1.5Ni0.5O4 with acid (Table II) reveals that Reaction 2 remains completely towards the left without the formation of any Mn3+ in the initial and delithiated samples. In other words, the Ni2+/3+ and Mn3+/4+ bands do not overlap in these spinel oxides as depicted in Figure 6, which is consistent with the neutron scattering and computational calculation data found in the literature34–36. The compositional analysis data in Table II are also consistent with a lying of the Mn3+/4+ energy well above that of the Co3+/4+ energy as depicted in Figure 6.
Figure 6. Qualitative energy diagram depicting the relative positions of the Mn3+/4+, Co2+/3+, and Ni2+/3+ redox energies relative to the top of O2−:2p band.
Conclusions
Extraction of lithium from four series of spinel oxides, LiMn2-xNixO4 (0 ≤ x ≤ 0.5) and LiMn2-xMxO4 (0 ≤ x ≤ 1.0) (M = Cr, Fe, and Co), with acid has been investigated systematically with an aim to establish the lithium extraction mechanism. XRD and chemical-compositional analysis data establish that the lithium extraction from spinel oxides occurs by a disproportionation of Mn3+ ions into Mn4+ and Mn2+ ions, with the latter being leached out into the solution, as was proposed by Hunter in 1981. The amount of lithium extracted is proportional to the amount of Mn3+ in the initial samples. The chemical-composition analysis of the delithiated samples in the four series of samples also reveal that other M3+ (M = Cr, Fe, and Co) ions in spinel oxides do not disproportionate into M4+ and M2+ ions unlike the Mn3+ ions. Furthermore, the data indirectly establish that the Mn3+/4+ band does not overlap with the Ni2+/3+ band, so in mixed oxides the Mn4+ + Ni2+ ↔ Mn3+ + Ni3+ equilibrium (Reaction 2) lies completely towards the left and no Mn3+ is formed. Moreover, ion exchange of Li+ ions by H+ ions does not occur in spinel oxides as the H+ ions may not be stabilized in tetrahedral sites of the spinel framework, while such ion exchange is known to occur readily in layered LiMO2 oxides, as the H+ ions may be readily stabilized in octahedral sites of the lithium layer in layered oxides.
Since only Mn3+ disproportionates into Mn4+ and Mn2+ while the other transition-metal ions do not, the study suggests that Mn dissolution could become much more pronounced than other transition-metal-ion dissolutions in mixed-metal oxide cathodes in the presence of trace amounts (ppm levels) of acid (e.g., HF) in the electrolyte when the cells are operated within the electrolyte stability region of <4.3 V vs. Li/Li+. However, at higher operating voltages (>4.3 V vs. Li/Li+), the cathode surface could become unstable in contact with the organic liquid electrolyte, which could lead to the dissolution of other metal-ions as well, even when no Mn3+ ions are present in the samples. Moreover, the chemical instability of cobalt-rich compositions due to an overlap of the Co3+/4+:3d band with the top of the O2−:2p band (Figure 4) could further exacerbate the metal-ion dissolution since the oxidation of O2− ions at higher operating voltages of >4.3 V in the Co-containing samples16 could promote the chemical reactivity and side reactions of the cathode with the electrolyte. Although the present work has been carried out at a much higher concentration of acid than would be encountered in an actual lithium-ion cell, the results clearly provide a trend and could provide insights in designing high-performance cathodes for lithium-ion batteries.
Figure 4. XRD patterns of LiMn2-xCrxO4 series before and after delithiation with acid. The expanded region over a small 2θ range on the right reveals the shifts in the peaks to higher angles upon delithiation. (a) x = 0 before delithiation, (b) x = 0 after delithiation, (c) x = 0.25 before delithiation, (d) x = 0.25 after delithiation, (e) x = 0.5 before delithiation, (f ) x = 0.5 after delithiation, (g) x = 0.75 before delithiation, (h) x = 0.75 after delithiation, (i) x = 1 before delithiation, and (j) x = 1 after delithiation.
Acknowledgments
This work was supported by the National Science Foundation Materials Interdisciplinary Research Team (MIRT) grant DMR-1122603 and Welch Foundation grant F-1254.