Abstract
Low voltage Polymer Ta capacitors fabricated with porous Ta anodes, anodic oxide films of Ta as a dielectric, and poly(3,4-ethylenedioxythiophene) (PEDOT) cathodes were investigated. The polymer cathodes were formed by either pure in-situ polymerization or hybrid polymerization, with in-situ PEDOT inside the porous anodes and a pre-polymerized PEDOT cathode on the external surface of the anodes. Anomalous transient current, DC leakage, and breakdown voltage were observed and investigated at normal and reverse polarities in a broad range of temperatures and voltages. Anomalously high transient current in the ampere range was observed when a pulse of rated voltage at normal polarity (+ on the Ta anode) was applied to the capacitors, and anomalous DC current was observed when rated voltage was applied at low temperature. This effect was significantly more pronounced in the hybrid devices, but significantly decreased in all cases with humidification. The anomalous currents were explained by the presence of dipoles, charged polymer chains, in the conducting polymer cathode at its interface with the dielectric.
Export citation and abstract BibTeX RIS
Polymer Ta capacitors are broadly used in modern electronics due to a combination of high charge efficiency and low equivalent series resistance (ESR), which allows high frequency applications and increased ripple current capability. These capacitors consist of a Ta anode sintered from Ta powder, an anodic oxide film of Ta as a dielectric, and an intrinsically conductive polymer cathode typically made of poly(3,4-ethylenedioxythiophene) (PEDOT).1–3
Recent work has shown that when Polymer Ta capacitors were manufactured with a flawless dielectric (no pores, cracks, crystalline inclusions) using a pre-polymerized PEDOT dispersion instead of a more traditional in-situ polymerization process, these capacitors demonstrated very low d.c. leakage (DCL), as well as record high breakdown voltage (BDV), working voltage (WV), and charge efficiency CV/cm3.4,5 Electrical properties of these capacitors were explained within the classical MIS (Metal-Insulator-Semiconductor) model, where in this particular case M corresponds to the Ta anode, I corresponds to the anodic oxide of Ta, Ta2O5, as insulator, and S corresponds to the PEDOT cathode, which acts as a p-type semiconductor. The key point of this theory with regard to Polymer Ta capacitors with a pre-polymerized PEDOT dispersion is that at normal polarity (+ on the Ta anode) the potential barrier at the IS interface effectively increases with applied voltage, limiting leakage current through the capacitor. In contrast, in Polymer Ta capacitors with in-situ PEDOT obtained by chemical reaction between monomer and oxidizer, surface charge at the IS interface pins this potential barrier, resulting in significant leakage current as the applied voltage increases. The surface charge at the IS interface was attributed to residuals of the in-situ polymerization reaction, while with the pre-polymerized PEDOT dispersion there is no chemical reaction involved.
Application of the pre-polymerized PEDOT dispersion by dipping and drying of the sintered and formed Ta anodes simplifies and intensifies manufacturing of Polymer Ta capacitors in comparison to the in-situ PEDOT with its numerous chemical reactions and subsequent washing and reformation steps. Furthermore, Polymer Ta capacitors with pre-polymerized PEDOT dispersion also demonstrate long-term stability and reliability due to a self-healing mechanism based on separation of PEDOT and the dopant [poly(styrene sulfonate)] (PSS) in local spots on the dielectric surface with increased current density.4 Both underdoped PEDOT and PSS have low conductivity, which means that their separation results in local non-conducting areas in the cathode blocking the current through defect spots in the dielectric.
Improvements in the performance, reliability, and manufacturing process of high voltage Polymer Ta capacitors with a pre-polymerized PEDOT dispersion stimulated interest in using pre-polymerized PEDOT dispersions in low voltage Polymer Ta capacitors as well. In comparison to high voltage Ta capacitors, low voltage Ta capacitors with thinner dielectrics are typically manufactured with finer Ta powder to increase the surface area of the anode and thereby the specific charge of the capacitor, CV/cm3. The high specific surface area and relatively small pore structure make it very difficult to impregnate these anodes with the pre-polymerized PEDOT alone. That is why in-situ PEDOT is still needed for internal impregnation of the porous anodes of low voltage Polymer Ta capacitors. However, the pre-polymerized PEDOT dispersion is applicable as an external part of the cathode in these capacitors.
This paper is dedicated to low voltage Polymer Ta capacitors with in-situ PEDOT and hybrid (in-situ internal PEDOT and pre-polymerized external PEDOT) polymer technology. This hybrid technology simplified and intensified the manufacturing process of low voltage Polymer Ta capacitors; however, unexpected performance problems were revealed in these devices. Particularly, a very high transient current was detected when a short voltage pulse was applied after surface mounting of these capacitors on a circuit board. This anomalous transient current did not cause any detectable permanent damage to the dielectric; it decreased with repetition of the voltage pulses as well as after exposure of the capacitor to a humid environment. In addition, anomalously high DCL was detected in these capacitors at low temperatures, while DCL was much lower at room temperature and even at elevated temperatures. In this paper, anomalous transient current, DCL, and BDV in low voltage Polymer Ta capacitors were investigated at normal and reverse polarities of the applied voltage in a broad range of temperatures and voltages. Experimental results were compared between hybrid PEDOT based capacitors and pure in-situ PEDOT based capacitors. Further development of the MIS model was performed to explain the experimental data.
Experimental
Capacitor fabrication
Tantalum powder with a specific charge of 150,000 μC/g and an average primary particle size of 0.9 μm was pressed into rectangular pellets of 2.5 mm × 2.5 mm × 4 mm with 5.5 g/cm3 green density. The pellets were sintered in vacuum at 1350°C for 20 min. A tantalum lead wire was embedded into the anodes during the powder pressing. Ta anodes were anodized in an aqueous solution of 0.1% of phosphoric acid at 80°C. The anodization included a constant current stage, I ≈ 1 mA/cm2, when the voltage increased from zero to the formation voltage, Vf = 18 V, and a constant voltage stage when the current was gradually decaying with time for three hours. The thickness, t, of this anodic oxide film was calculated to be 44.7 nm using the equation t = 3.3 + 2.3 Vf.6 In-situ oxidative polymerization of PEDOT was performed by polymerization of 3,4-ethylenedioxythiophene (Baytron M from H. C. Stark) in the presence of iron (III) toluenesulfonate with a monomer/dopant ratio of 3:1. Good coverage of the dielectric with the cathode material in the core of the anodes was confirmed directly by SEM analysis of the fractured anodes and also by small changes (below 5%) between capacitance measurements in liquid electrolyte and with a dry solid cathode. A pre-polymerized PEDOT cathode was applied by dipping the Ta/Ta2O5 pellets into a water-born dispersion of the nano-scale PEDOT particles (Clevios Knano) and subsequent drying in air at room temperature and then at 150°C for approximately 30 minutes.5 Using an ultracentrifuge technique, H. C. Starck determined that 50% of the PEDOT particles in the dispersion had a diameter of d ≤ 26 nm. The dipping and withdrawal were perpendicular to the dispersion surface with a rate of 0.5 mm/s and a hold time of 30 seconds. This pre-polymerized PEDOT contains the dopant [poly(styrene sulfonate)] (PSS), which provides a high p-type conductivity to the PEDOT-PSS cathode. In-situ oxidative polymerization of PEDOT was performed by polymerization of 3,4-ethylenedioxythiophene (Baytron M from H.C. Stark) in the presence of iron (III) toluenesulfonate with a monomer/dopant ratio of 3:1.4. A carbon layer obtained from a coating solution comprising graphite particles dispersed in polyester, and a silver layer obtained from silver paste which included Ag particles with an average diameter of about 10 μm, an acrylic binder, and diethylene glycol monoethyl ether (DE) acetate as solvent, were used to connect the PEDOT cathode to the external negative termination of the capacitors. Variations in the actual capacitance readings on 20 samples were within ± 5% of the average capacitance, C = 463 μF, measured at a frequency of 120 Hz with no DC bias applied.
Electrical measurements.—Transient current measurements
To observe the current drawn by the capacitor immediately after the application of a voltage pulse, a Digital Storage Oscilloscope (DSO) was used. A circuit employing a TIP 120 NPN Darlington Pair was used to pulse the capacitor, and an Arbitrary Waveform Generator (Tektronix AFG3021) was used to generate the test signals. Both the applied voltage pulse across the capacitor and the current passing through the capacitor were monitored and recorded. To observe the current, the voltage developed across a 0.1Ω resistor placed in series with the capacitor was measured on the DSO. In addition, a 200Ω resistor was also placed across the capacitor to facilitate quick capacitor voltage discharge after the test. A schematic of this arrangement is shown in Fig. 1. Screenshots from the DSO were captured over an Ethernet connection and were subsequently digitized.
Figure 1. Schematic of the circuit used to pulse the Polymer Tantalum capacitors.
DC leakage current measurements
Capacitors were soldered on to a test board to perform measurements on several parts in parallel. To measure the DC Leakage, a voltage was applied across the capacitor and a 100Ω series resistor. The value of current was measured by monitoring the voltage across this 100Ω resistor using a Data Acquisition System, HP 3421A, which was controlled by a PC. The current was allowed to decay for 300 seconds before its final value was recorded. Such measurements were repeated at various voltages to get a complete I-V curve for the device. Measured curves of several devices were averaged to obtain a representative I-V curve for each capacitor type. Measurements at an elevated temperature were performed by placing the test capacitor board in a temperature-controlled oven; measurements at Liquid Nitrogen (LN2) temperature were performed by submerging the test capacitor board in a flask of LN2.
C-V measurements
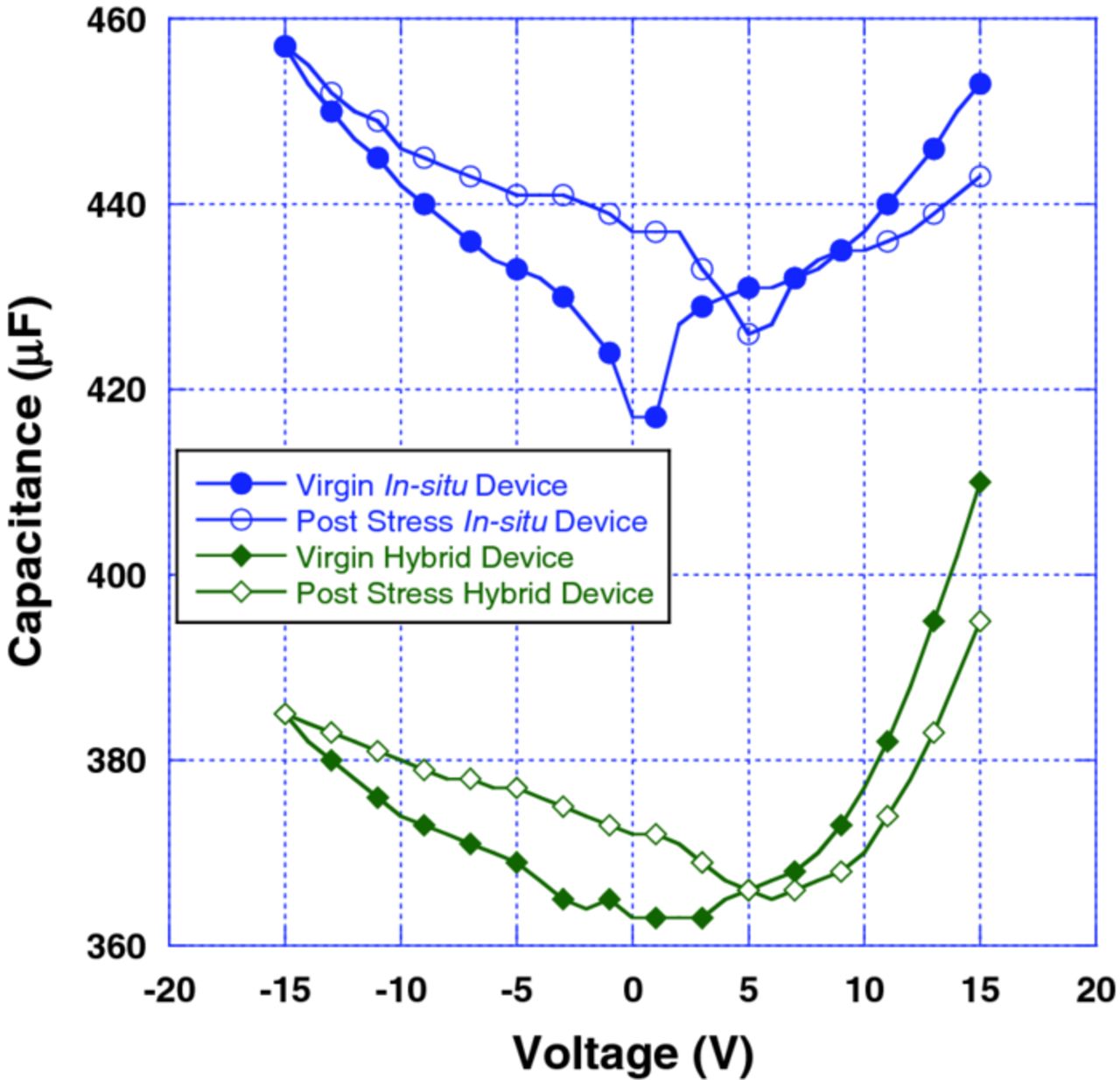
C-V measurements were performed using an Agilent Precision LCR meter, E4980A. Capacitance measurements were performed at a test signal frequency of 120 Hz at a level of 1 V. Bias voltage was applied automatically using a built-in DC Bias Sweep function. C-V measurements were initially performed on virgin devices in the bias range of +15 to –15 V, at LN2 temperature in order to limit reverse leakage current. Subsequently, the capacitors were allowed to warm up to room temperature, after which the devices were stressed by applying a +5 V DC bias across the terminals for 1 minute. The capacitors were subsequently cooled under bias back down to LN2 temperature and the C-V measurement was repeated. Several capacitors were characterized in this manner to obtain a representative behavior. C-V results before and after the stress were compared in order to look for a shift in flatband voltage. Such a shift indicates the presence of charge inside the capacitor.
Breakdown voltage measurements
Breakdown Voltage (BDV) measurements were performed at room temperature on a group of 20 hybrid capacitors and a group of 20 in-situ capacitors, with a 1A fuse in series with each capacitor. Voltage was increased at a constant rate of 0.5 V/min from 0 V to the voltage at which the fuse opened or to a maximum value of 25 V, which was approximately 50% higher than the capacitor's formation voltage. A breakdown event was registered by either a blown fuse (catastrophic breakdown) or by a short spike in current and a drop in voltage (scintillation breakdown). The deviation from the average BDV within a group of 20 capacitors was within +/−1 V.
Thermal analysis
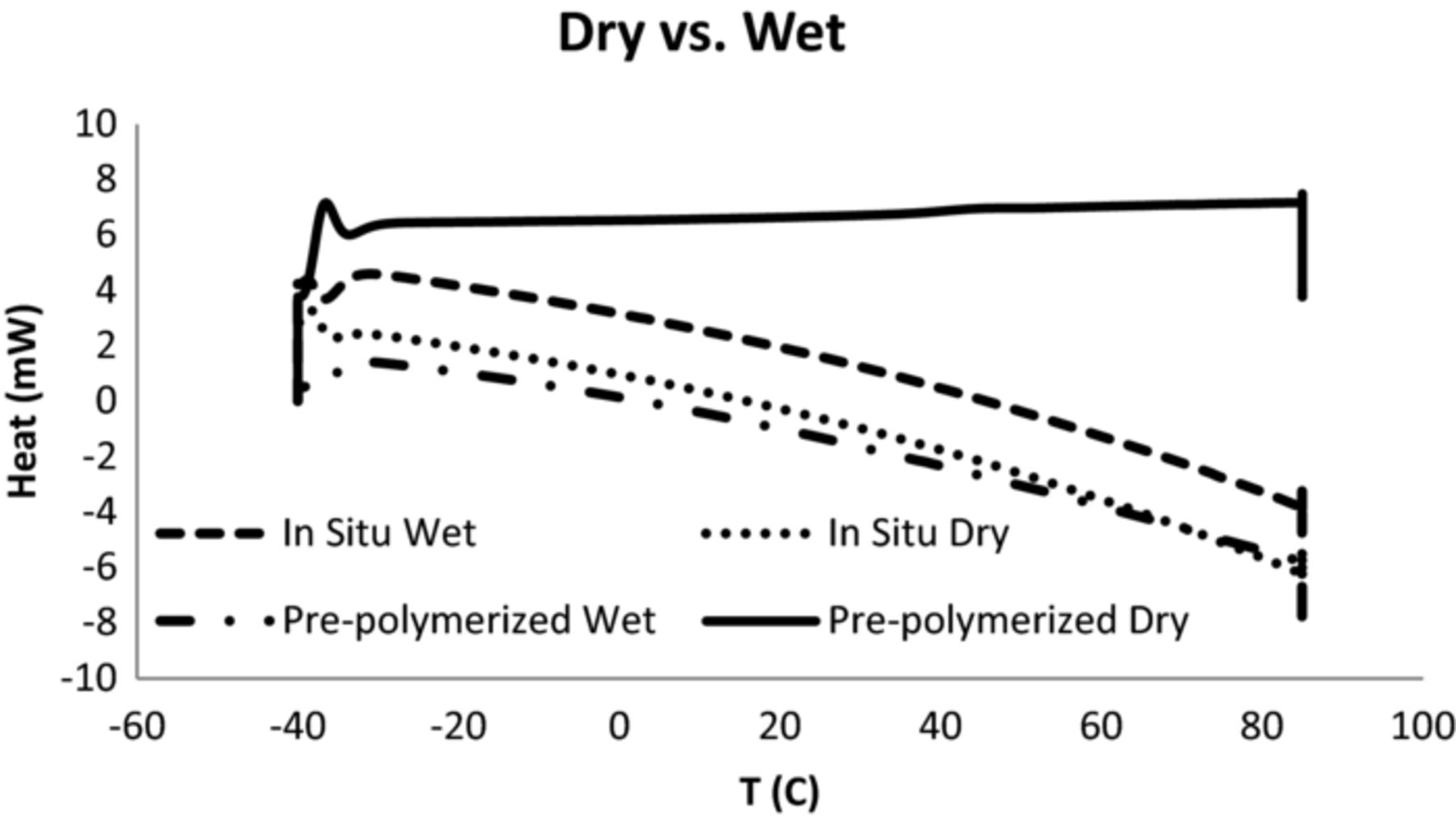
Effects of humidity on the phase transformations in both in-situ and pre-polymerized PEDOT polymers were studied using a Perkin Elmer DSC7, (Differential Scanning Calorimeter). Samples were initially cooled from room temperature to –40°C. The samples were then heated to 85°C, at a heating rate of 20°C/min, and the heat flow changes were measured against a blank pan as a function of temperature. The effect of moisture was studied by either humidifying or drying the samples. Polymer samples were dried at 125°C for 24 hrs, from here onward referred to as dry samples. For humidification, the polymer samples were exposed to 85% relative humidity at 85°C for 24 hrs., and from here onward referred to as wet samples. In both cases (dried/wet), samples were closed in air tight holders inside the ovens and then transferred to a glove bag. Samples for DSC were prepared inside the glove bag as well to avoid any moisture change.
Results and Discussion
Characteristics of hybrid Polymer Ta capacitors
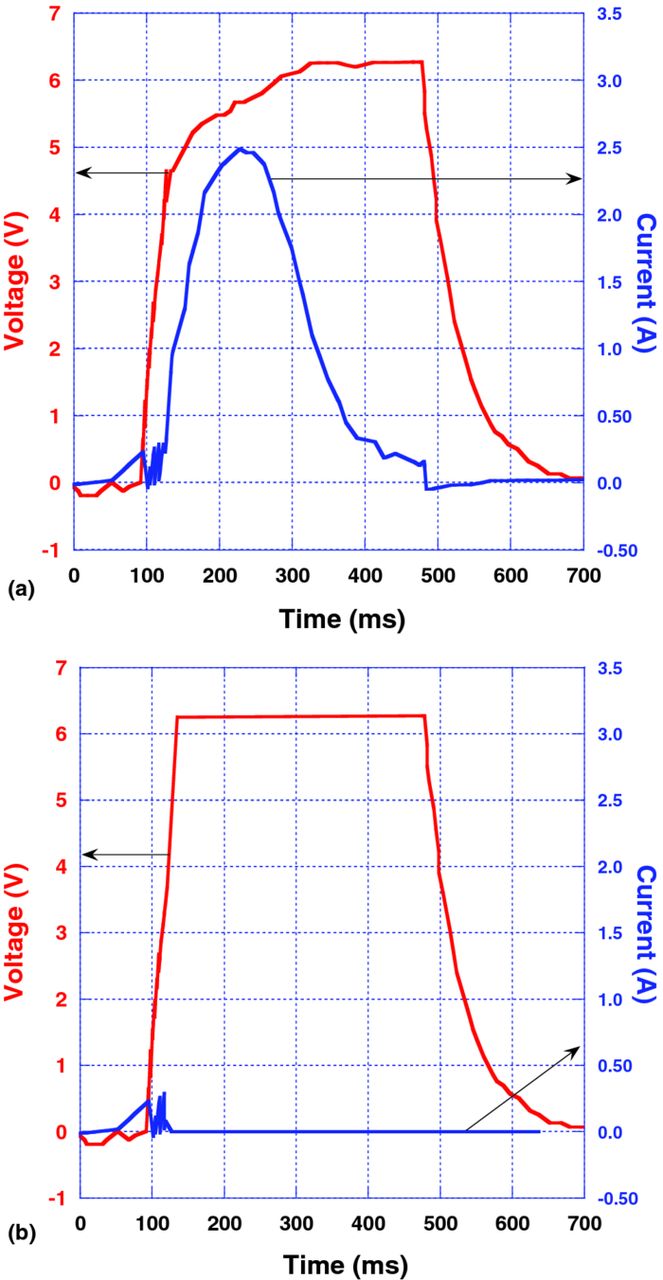
Fig. 2 presents I(t) and V(t) characteristics for the first pulse (a) and repeated pulses (b) at rated voltage and room temperature for a 470 μF–6.3 V hybrid Polymer Ta capacitor. As one can see from Fig. 2a, current begins increasing sharply after a certain delay when voltage is applied. This anomalous transient current reaches a maximum value in the ampere range and then decays to the milliamp range within a fraction of a second. The smaller spikes due to pure displacement current can be seen at the beginning of the pulse. The magnitude of transient current decreases after each pulse and ultimately becomes negligible after repeated testing, as seen in Fig. 2b. The behavior demonstrated by the hybrid polymer capacitors suggests that polar polymer chains constituting the cathode respond when the charge pulse is applied. Indeed, it is well known in polymer science that polar groups on the polymer chains act in response to an electric field.7 Therefore, when the field is applied to the capacitor, PEDOT and PSS macromolecules carrying strong dipoles reorient accordingly. This reorientation occurs with a certain rate connected to the mobility of the polymer segments. If the reorientation rate is significantly lower than the rate of the charge application, an anomalously high current is observed during the first pulse. Since application of the following pulses does not result in significant current, it can be concluded that the chains are settled in their new quasi-permanent positions after the very first pulse.
Figure 2. (a) (Color online) I(t) response to one pulse, V(t), applied at room temperature to a 470 μF–6.3 V hybrid Polymer Ta capacitor: (b) (Color online) I(t) response to a pulse, V(t), after repeated pulses applied at room temperature to a 470 μF–6.3 V hybrid Polymer Ta capacitor.
The response rate of the polymer chains can be regulated by control of the polymer chain mobility.7 First of all, the rate can be increased by the addition of small molecules, plasticizers that dissolve in the polymer, separating the chains from each other and hence making the chain movement easier.7 Secondly, the chain mobility can be significantly decreased by decreasing the temperature of the sample. In a subsequent experiment, we exposed the capacitors to the air with a relative humidity of about 70%. It was determined that the polymer cathode absorbed an amount of water equal to about 8% of the dry polymer weight. In fact, no measurable anomalous current was detected in capacitors after the plasticizing water molecules were introduced into the system. Drying of the capacitors at 125°C for 24 hrs., restored the anomalous transient current similarly to that shown in Fig. 2a. DCL in these capacitors measured after rated voltage was applied for 90 sec at room temperature was low and practically identical before and after voltage pulses were applied.
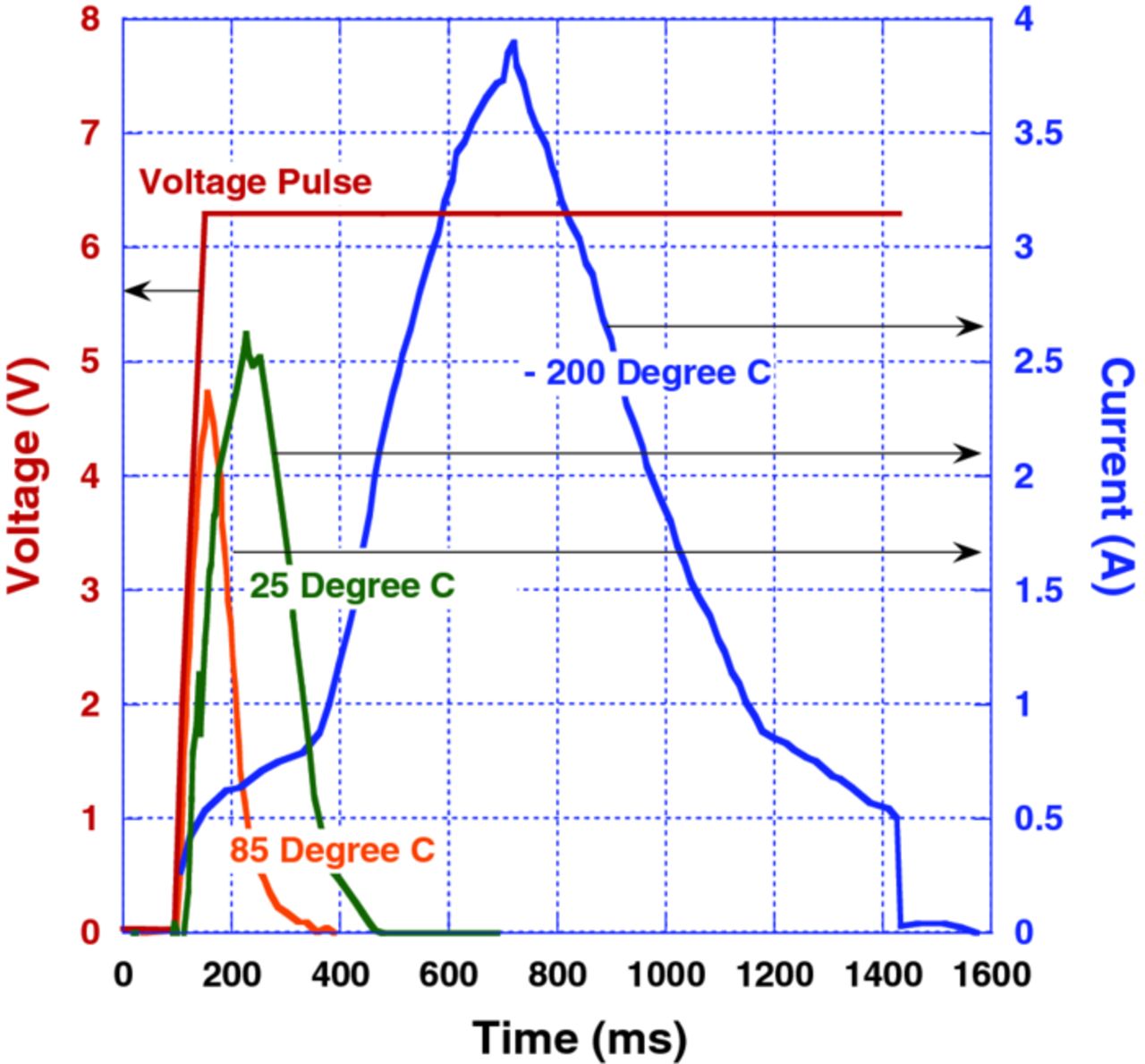
Fig. 3 shows the effect of temperature on anomalous transient current in 470 μF–6.3 V hybrid Polymer Ta capacitors. According to Fig. 3, the time delay at the beginning of the transient current increase, the magnitude of the peak current, and the amount of charge corresponding to the transient current (area under the current curve) - all increase with decreasing temperature. The highest magnitude of the transient current as well as the amount of the corresponding charge were detected at –200°C, after the sample was cooled by liquid nitrogen. Decreasing temperature also alters the waveform characteristics of the transient current.
Figure 3. (Color online) Effect of temperature on anomalous transient current, I(t), in response to a voltage pulse, V(t), applied to a 470 μF–6.3 V hybrid Polymer Ta capacitor.
Fig. 4 shows a series of consecutive pulses of rated voltage at –200°C applied at normal polarity (Fig. 4a, Fig. 4b, and Fig. 4d) and reverse polarity (Fig. 4c). The first pulse at normal polarity, which was applied after the capacitor was dried at 125°C and cooled to –200°C by liquid nitrogen (Fig. 4a), shows high anomalous transient current. The magnitude of the transient current becomes negligible after repeated testing (Fig. 4b). At reverse polarity (Fig. 4c) the current is so high that the voltage on the capacitor is reduced, partially due to redistribution of some of the applied voltage to the series resistor used in the measurement circuit and partially due to overload of the power supply at such high currents. The next pulse at normal polarity following the pulse at reverse polarity (Fig. 4d) shows again high transient current similar to that shown in Fig. 4a. From these data, we observe that high anomalous transient current in Polymer Ta capacitors, which diminishes after repetitions of pulses at normal polarity, can be restored by a short application of reverse voltage without long-term drying at high temperatures. It is evident that application of a reverse bias causes the migration of the polymer segments to their initial (prior to the first pulse) positions as indicted by the significant current observed. It is noteworthy to point out that in our earlier work on high voltage polymer Ta capacitors with pre-polymerized PEDOT cathodes, we did not observe any of these anomalous effects.5 However, in this earlier work the cathode was pure pre-polymerized PEDOT, the Ta powder was coarser, and the oxide was more than four times thicker, resulting in essentially different materials. Low Voltage Polymer Ta capacitors with pure pre-polymerized PEDOT cathodes are currently under investigation in our laboratory in order to yield further insight into these anomalous effects.
Figure 4. (Color online) Effect of consecutive pulses at –200°C applied to a 470 μF–6.3 V hybrid Polymer Ta capacitor. (a) Initial pulse at Normal Polarity, V(t), and current response, I(t): (b) (Color online) I(t) response to a pulse, V(t), after repeated pulses at Normal Polarity: (c) (Color online) Subsequent I(t) response to a pulse, V(t), at Reverse Polarity, after repeated pulses at Normal Polarity: (d) (Color online) Final pulse at Normal Polarity, V(t), and current response, I(t).
Comparison of hybrid and pure in-situ Polymer Ta capacitors
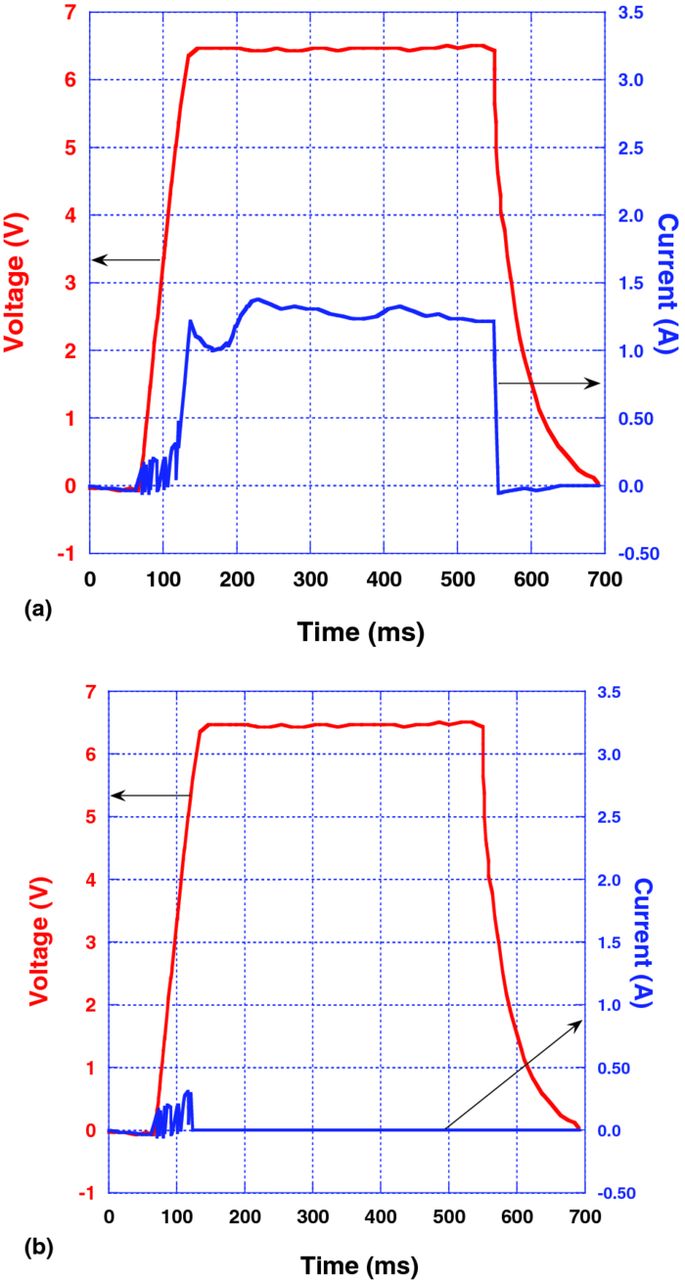
Fig. 5 shows the pulse characteristics of 470 μF–6.3 V Polymer Ta capacitors with hybrid (a) and pure in-situ (b) cathodes. These characteristics were obtained after drying the capacitors at 125°C for 24 hrs. and then cooling them to –200°C in liquid nitrogen. As one can see, anomalous transient current is observed at rated voltage only in hybrid capacitors, while there is practically no anomalous transient current in Polymer Ta capacitors with pure in-situ PEDOT cathodes.
Figure 5. (a) (Color online) I(t) response to one pulse, V(t), applied at –200°C to a 470 μF–6.3 V hybrid Polymer Ta capacitor: (b) (Color online) I(t) response to one pulse, V(t), applied at –200°C to a 470 μF–6.3 V pure in-situ Polymer Ta capacitor.
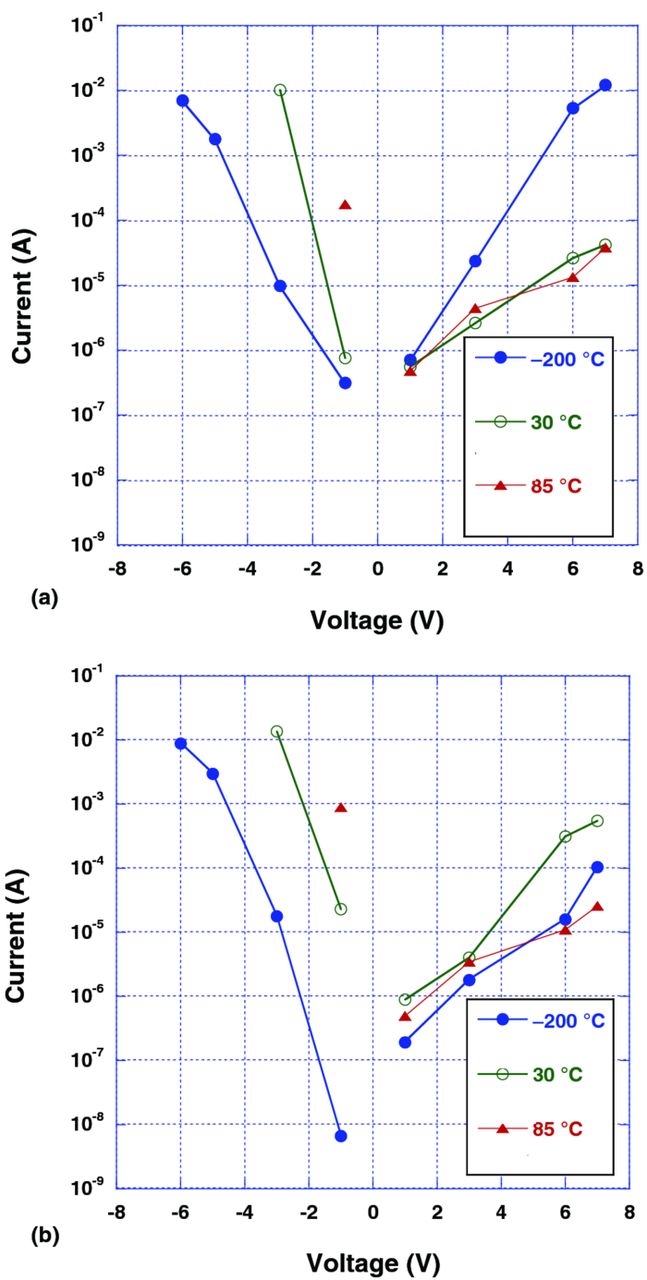
Fig. 6 presents I-V characteristics of Polymer Ta capacitors with hybrid (a) and pure in-situ (b) PEDOT cathodes. In hybrid capacitors (Fig. 6a) at normal polarity, anomalously high DCL was detected at –200°C. The difference between DCL at –200°C and DCL at room temperature and 85°C, is increasing with voltage. The high current in the range of several milliamps was observed in the capacitors for several hours as long as the capacitors remained submerged in LN2 and rated voltage at normal polarity was applied. However, in capacitors with pure in-situ cathodes (Fig. 6b), DCL at normal polarity is lower at –200°C in comparison to DCL at room temperature and 85°C. Both hybrid PEDOT and in-situ PEDOT types of capacitors demonstrate polar behavior with low current at normal polarity in comparison to the much higher current at reverse polarity.
Figure 6. (a) (Color online) I-V curves of 470 μF–6.3 V hybrid Polymer Ta capacitor at –200, 30, and 85°C: (b) (Color online) I-V curves of 470 μF–6.3 V pure in-situ Polymer Ta capacitor at –200, 30, and 85°C.
We suppose that the difference in the behavior between the hybrid and in-situ parts is connected to the possibility for the polymer chains, constituting the pre-polymerized material, to reorient more slowly and at more significant levels when the electric field is applied. The reorientation influences the band structure at the interface, where the mobile dipoles orient themselves according to the field. When the PEDOT-PSS sample is humidified, the reorientation happens more quickly, since the water works as a plasticizer for the polymer chains involved.7 It is also important to note that when water is present, the PSS monomeric units, which are not associated with PEDOT, can dissociate to create SO3− ions connected to the polymer chain and free H+ protons.8 When the sample is dry, the movement/reorientation of the dipoles becomes slower, which results in the significant anomalous current which is observed. A decrease in temperature decreases the mobility of the dipoles as well. For the in-situ parts there are no polar PSS macromolecules present. In this case only low molecular weight paratoluene sulfonic acid (pTSA) is associated with PEDOT units. There is practically no significant amount of "free" acid groups in the system. Since PEDOT decorated with pTSA is not soluble in water and free acid groups are absent, the presence of the water molecules should have a much smaller impact on the behavior of in-situ capacitors vs. hybrid ones.
Thermal analysis of in-situ and pre-polymerized materials supports our suggestions. Fig. 7 shows the effect of moisture on the phase transformations in the in-situ and pre-polymerized PEDOT polymers. No endothermic or exothermic peaks were observed in any of the samples, suggesting no significant phase transformation occurred in any of these polymers in this temperature range. A closer examination of Fig. 7 reveals that the in-situ polymer shows very similar behavior irrespective of moisture level, since both dry and wet curves have similar negative slopes. For the case of the pre-polymerized samples however, moisture did have a significant impact. The wet sample behaved in a very similar fashion as the in-situ sample, whereas the dry sample showed almost no difference in heat-flow rate as a function of temperature. It appears that for the pre-polymerized PEDOT-PSS samples, in a dry condition the presence of long chain PSS molecules makes motion of the polymer chains somewhat restricted. Hence, heat capacity increases with temperature in the temperature range studied. This indicates that energy is consumed to support the onset of segmental diffusion of the macromolecules. The presence of H2O makes motion of these molecules possible at the lower temperatures and therefore heat capacity is relatively constant in the temperature range studied. In the case of in-situ polymers, the presence of low molecular weight p-TSA acts as a plasticizer, and therefore humidity does not appear to make a significant difference in this temperature range.
Figure 7. Effect of moisture on the heating behavior of in-situ and pre-polymerized PEDOT.
Fig. 8 shows the typical C-V characteristics of Polymer Ta capacitors with hybrid and pure in-situ cathodes. In both types of capacitors, a shift in the flatband voltage was observed after room temperature voltage stress was applied. This indicates the buildup of charge inside the capacitor structure. The generation of this charge can be attributed to the reorientation of the charged polymer chains due to the applied voltage. Motion of mobile ions inside thin films of SiO2 has been shown to produce such flatband shifts in MOS capacitors; however, these ions move within the dielectric layer very slowly, and normally require application of both elevated temperature and an electric field to observe effects in a reasonable amount of time.9 Typically, at least 5 to 10 minutes of biasing at temperatures on the order of 200°C is required before mobile ions produce noticeable differences in C-V curves. In experiments with these capacitors, bias voltages applied even for a few seconds at room temperature can result in a significant shift in C-V curves indicating that the charge generating mechanism allows for a quick response. Therefore, the shifting of the C-V curves is not likely due to ion transport in the film; instead, it can most likely be attributed to polarization of the charged polymer chains under the applied electric field. Upon shorting the capacitors for a few seconds at room temperature, the C-V curves shift back to their original shape. Similar shifts in C-V curves were observed in several conducting polymer capacitors developed by other manufacturers, indicating that this phenomenon can be present in various organic devices in general.
Figure 8. (Color online) C-V curves of the 470 μF–6.3 V Polymer Ta capacitors, hybrid and pure in-situ.
Finally, I-V and C-V characteristics of in-situ and hybrid capacitors during BDV testing are shown in Fig. 9a and Fig. 9b respectively. These results show that breakdown for in-situ capacitors was characterized by a rapid current increase and capacitance loss. Furthermore, these capacitors effectively became shorts and the fuse was blown. For hybrid capacitors however, a scintillation breakdown was observed as a short current spike and a drop in voltage, followed by a relatively low current and gradual decrease in capacitance as applied voltage was increased. The different types of breakdown observed for in-situ and hybrid capacitors can also be explained by the differences in their polymer cathodes. At extreme voltages, approaching BDV, PSS chains in the hybrid capacitors can separate from the PEDOT chains and form an additional dielectric layer on top of the tantalum oxide dielectric.4 This results in a loss of capacitance and relatively low leakage current even when the applied voltage exceeds the formation voltage. In-situ capacitors become shorts since they don't have the ability to block rapidly increasing current by forming a new dielectric on top of the degrading oxide dielectric.
Figure 9. (a) (Color online) I-V curves during BDV testing of the 470 μF–6.3 V Polymer Ta capacitors, hybrid and pure in-situ: (b) (Color online) C-V curve during BDV testing of the 470 μF–6.3 V Polymer Ta capacitors, hybrid and pure in-situ.
Implications for Polymer Ta capacitors
The results we have presented in this paper have some significant implications for Polymer Ta Capacitors. According to our experimental results, there are some significant differences between the electronic properties of hybrid and pure in-situ capacitors. Anomalous transient current was observed in hybrid devices after drying, while a negligible amount of such current was observed in pure in-situ devices at or below rated voltage whether dry or humidified. In general, it was observed that in hybrid devices the anomalous transient current increased for dried devices, and it also increased at lower temperatures including peak current, total charge transported, and delay time between application of the pulse and the onset of the anomalous current. On the other hand, the anomalous transient current decreases with humidity, repeated application of pulses, pulse width, and a slower ramp rate of the applied pulse. These essential observations can be explained by a charge polarization mechanism in the devices connected to the mobility of polymer chains constituting the cathode material.
The presence of dipole layers is well known in conducting polymers and organic devices.10 The dipoles can produce significant effects on the electronic properties of materials and devices; and furthermore, these dipoles are influenced by an applied electric field. An electric field applied to a material system will orient the dipoles according to the direction of the field, and this determines their dipole moment. If the electric field is suddenly switched off, such as with a pulse, these dipoles will tend to randomize; i.e., their dipole moment, or their polarization, will go to zero. However, this relaxation cannot occur instantaneously; it is a collision process with a characteristic relaxation time, or time constant, t.11 The relaxation time is a function of temperature and the process can be accelerated by elevated temperatures. In a polymer these dipoles are primarily due to charged polymer chains, which will reorient when a field is applied. This reorientation is more pronounced in pre-polymerized cathodes, and therefore in the hybrid devices, compared to pure in-situ cathodes. The reorientation of the dipoles can significantly influence the insulator–semiconductor interface of the MIS structure, where the mobile dipoles orient themselves with the field.10,12–14 In humidified samples the reorientation is faster than the process in dry devices, since the water acts as a plasticizer for the polymer chains. Therefore in dry samples, where the reorientation is slower, there is more time to significantly affect the interface.
Dipoles at the dielectric/semiconductor interface of an organic MIS device can affect the barrier significantly.10,12,14,15 The energy bands can be deformed, altering the barrier height and pinning the Fermi level.14 Leakage current can increase or decrease depending on dipole direction which is determined by the polarity of the applied field. In this work a pulse was applied, resulting in an anomalous transient current which will decay during the dipole relaxation process after the pulse ends, eventually canceling the effect. As temperature decreases, the dipole relaxation time increases while the mobility decreases. This explains why we observe a higher transient current which flows for a longer time at lower temperatures, as shown in Fig. 3 where the peak current and area under the I-time curve is larger at –200°C. The results for DC leakage are also consistent with this mechanism, as DCL measured after 300s of stress at rated voltage is higher at lower temperatures and for dry samples. Therefore anomalous transient and DCL currents are the most pronounced in Polymer Ta capacitors with dry hybrid cathodes. This is due to the lack of a plasticizer in dry polymers, the charged polymer chains in the pre-polymerized PEDOT, and the presence of PSS which slows the dipole relaxation process. Finally, it is important to emphasize that the primary effect of the dipoles on these capacitors is to modify the barrier, which governs charge transport through the MIS structure; however, the current transport through the device is predominantly electronic.5
Conclusions
In this paper we describe anomalous currents in low voltage Polymer Ta capacitors having porous Ta anodes, anodic oxide films of Ta employed as a dielectric, and a conductive polymer cathode. Anomalous currents include anomalous transient current when a pulse of rated voltage at normal polarity (+ on the Ta anode) is applied to the capacitors, and also anomalous DC current when rated voltage is applied to the capacitors at low temperature. Anomalous currents are more pronounced in Polymer Ta capacitors with hybrid cathodes, having an in-situ PEDOT cathode inside porous anodes and a pre-polymerized PEDOT/PSS cathode on the external surface of the anodes. Anomalous currents decrease with the humidification, but they can be restored by drying the capacitors or by application of the voltage pulse at reverse polarity (–on Ta anode).
The anomalous currents observed in Polymer Ta capacitors were explained by the presence of dipoles, charged polymer chains, in the conducting polymer cathode at its interface with the oxide dielectric. These dipoles will reorient when an electric field is applied, and this reorientation determines the potential barrier at the oxide-polymer interface. As presented earlier, this potential barrier plays a crucial role in limiting the current through the Polymer Ta capacitor. The dipole relaxation time is a function of temperature, and therefore the process slows down at lower temperatures when mobility of polymer chains becomes low. In humidified capacitors the reorientation is faster than the process in dry capacitors, since the water acts as a plasticizer for the polymer chains. This reorientation, and the resulting anomalous currents, is more pronounced in pre-polymerized cathodes with long molecules of PSS in the polymer structure; therefore, it is more pronounced in capacitors with the hybrid cathodes as compared to those with pure in-situ cathodes. The dipole relaxation process explains the restoration of anomalous currents after a characteristic relaxation time when voltage is not applied to the capacitors. The differences in the polymer structure in the capacitors with in-situ and hybrid cathodes can also explain the differences in their electric breakdown; in particular, scintillation breakdown in hybrid capacitors with resistance restoration is observed even as the applied voltage exceeds the formation voltage.
Acknowledgments
The authors thank KEMET's Dr. Erik Reed for introduction to the phenomena of anomalous currents in Polymer Tantalum capacitors and helpful discussions; as well as, Jonathan Paulsen and Steve Hussey for their assistance with the fabrication and electrical testing of the capacitors, and the help of Cynthia Prince in conducting the DSC analysis.