Abstract
Titania nanotubes (TiO2 NTs) of 5 μm in length and 100 nm in the external diameter are easily formed by anodic oxidation. They are used as hollow substrates to deposit different ZnO nanostructures, such as nanoparticles and nanowires by employing two different techniques, electrodeposition and hydrothermal growth, respectively. In this way highly nanostructured and hierarchical sample surfaces were obtained, showing high level of crystallinity of both TiO2 anatase and ZnO wurtzite materials. In addition, the wetting behavior drastically changed from the hydrophilic TiO2 NTs surface to almost superhydrophobic surfaces of the hierarchical samples, thanks to the decoration with ZnO nanostructures. These results open interesting possibilities to employ our hierarchical TiO2-ZnO nanostructured materials as self-cleaning, antireflective or anti-fogging surfaces. These hierarchical and composite nanostructures could be thus efficiently used in photocatalytic devices, which would also benefit from the combination of both metal oxides for improved performances and efficiencies.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives 4.0 License (CC BY-NC-ND, http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial reuse, distribution, and reproduction in any medium, provided the original work is not changed in any way and is properly cited. For permission for commercial reuse, please email: oa@electrochem.org.
Anodic oxidation1 is the process of forming an oxide on a metal surface by applying an electric potential through a suitable electrolyte. The metals that can be anodized belong to the so-called valve-metals group (Al, Ti, Zr, Nb, W, Ta, ...) and different kinds of ordered nanostructured oxides can be obtained. Compared with other synthesis approaches, electrochemical anodization is a simple and convenient technique to fabricate uniform layers of vertically self-oriented nanostructures. It has been widely accepted that the formation of the pores in anodic metal oxides is based on two continuous processes: the oxide dissolution at the electrolyte/oxide interface and the oxidation of metal at the oxide/metal interface.
Particularly, anodic titanium oxide has attracted considerable interest since its unique properties make it useful for various functional applications, ranging from non-silicon solar cells2–4 and photocatalysis5 to energy storage.6,7 In general, the morphology and the structure of the ordered layer are strongly affected by the electrochemical conditions (anodization voltage, distance between the electrodes, temperature) and the electrolyte composition. It is possible to grow titania nanotubes (NTs) with length varying between 100 nm up to 1 mm with a good control on the wall thickness and external roughness.
In the present paper we report on the anodic oxidation of a titanium foil to form 5 μm long titania NTs. In order to create a hierarchical structure and then to study the wetting properties, we decorate both their inner and outer surface with zinc oxide (ZnO) nanoparticles of about 20 nm in diameter. In particular, by using two different growth techniques, it is possible to additionally enrich the planar surface of the TiO2 NTs with either a network of ZnO nanowires using the hydrothermal approach, or with ZnO microparticles of about 150 nm using the electrodeposition method.
ZnO is a n-type metal oxide semiconductor having a wide bandgap, high electron mobility and high thermal conductivity. It mainly crystallizes in the wurtzite phase, being intrinsically polar, and thus leading to interesting piezoelectric properties. Additionally, ZnO possesses promising photocatalytic, electrical, electronic and optical properties. It can be easily prepared in different shapes and sizes at both the micrometric and nanometric scale using different synthesis techniques. Among these methods, the hydrothermal and the electrodeposition syntheses are mainly used for their easy, low-cost, and high throughput yield of ZnO micro- and nanostructures. The hydrothermal growth of ZnO consists in hydrolysis and condensation of a ZnO precursor in basic solutions8,9 leading to several micro- and nano-particles morphologies,10,11 including nanowires. In particular, this method can lead to an array of vertically oriented nanowires on whatever substrates after the deposition of a crystalline and oriented ZnO seed layer, as previously described.9,12,13 In this synthesis no additional heat or chemical treatments are required to achieve the wurtzite-type crystal structure of ZnO. In addition this method does not require high pressure containers and is also entirely recyclable, safe and environment friendly since water is used as solvent.14
The ZnO electrodeposition leads to different nanostructures, including nanowires and nanoparticles, on conductive substrates.15,16 During this process, an oriented diffusion of charged reactive species through the solution takes place under the application of an external electric field. The electrodeposition is a versatile and scalable method. It also works at low temperature, i.e. below 100°C, and the morphology of the nanostructure, such as diameter and length, can be easily tuned by varying the process conditions, such as voltage, time, charge density and precursor concentration.
The combination of ZnO and TiO2 is well documented in the previous literature17–19 as the formation of heterojunctions for photocatalysis. In particular, different constructs of ZnO-TiO2 composite materials have been presented, such as core-shell20 or branched structures,21 showing a superior photo-electrochemical activity due to the improved electron-hole separation and lower recombination of charge carriers. Such features are responsible for the enhancement in the efficiency of both Dye Sensitized Solar Cells (DSSCs)22 and Photo-Electro-Chemical (PEC) water splitting cells.20,23 For example, macro–meso-porous thick films of ZnO–TiO2 composites, having homogeneously distributed open porosity and a superhydrophilic behavior, were used as efficient photocatalyzers in the degradation of methyl orange.24 In addition hierarchical TiO2 and ZnO materials were recently presented25,26 as substrates for biomedical implants for bone and gingival tissue regeneration. In particular, Chu et al.25 studied the wetting behavior of porous titania micro-holes and groove structures covered by ZnO nanorods. They showed the role of ZnO in increasing the roughness of the surface and thus changing the wetting behavior of the hierarchical material, varying from the hydrophilic titania to the hydrophobic hierarchical TiO2-ZnO surface. However in the reported paper, water contact angle (WCA) values are missing and the sample wetting behavior is therefore only qualitatively discussed. In our study, WCA experiments are carried out on both hierarchical TiO2-ZnO samples, i.e. composed by the titania nanotube array covered by either ZnO nanowires or microparticles. These results are compared to those obtained for the pristine titania NTs sample, flat films of both TiO2 and ZnO, and to ZnO nanowires. In this way the contribution of both the nature of the material and its nanostructuration are decoupled in order to understand their role in the final wetting behavior. To our knowledge, no other paper reports on the construction of such hierarchical samples and the evaluation of their wetting behavior. The obtained findings show a dramatic change of the wetting behavior before and after the ZnO nanostructure deposition on the NTs samples, opening considerations about the influence of both the nature and the nanostructured morphology of such hierarchical samples on their wettability. In this way, highly and even superhydrophobic hierarchical TiO2-ZnO surfaces can be efficiently produced. These composites having two metal oxide nanostructures can be efficiently used as antifogging or self-cleaning surfaces, preventing dust deposition and allowing antireflective coatings. Thus they can successfully be employed in photovoltaic or photocatalytic applications,27–29 in which optical losses caused by dust deposition and light reflection at the surface may be reduced having a superhydrophobic surface made by ZnO NWs.30
Alternatively, a possible application in biomedical hard tissue implants can be also envisioned, where control on bone cell adhesion and proliferation can be optimized by varying the surface nanostructuration, even using hierarchical composite material surfaces.25
In this work we therefore aim to study the surface properties, and in particular the wetting behavior, of hierarchical TiO2-ZnO nanostructures, efficiently controlling their hydrophobic behavior.
Experimental
Synthesis of titania nanotubes
Titanium foils (thickness 250 μm, 99.96% purity, Goodfellow) were cut into 2 × 2 cm2 pieces and used as active substrates for the anodic growth of TiO2 nanotubes. The foils were cleaned by ultra-sonication in acetone and rinsed in ethanol. Anodic oxidation was conducted at 25 °C in an electrolytic solution containing 0.5 wt% NH4F (99.5%, Sigma Aldrich) and 2.5 vol.% deionized water in ethylene glycol (98%, Sigma Aldrich), using a platinum sheet as counter electrode (thickness 250 μm, 99.99% purity, Goodfellow). The anodization time was fixed to 15 min, working under continuous stirring with a constant voltage of 60 V provided by a DC power supply (GW Instek SPD-3606). At the end of each anodic oxidation, the samples were rinsed in DI-water and dried on a hot plate. Since the as-grown nanotubes are obtained in an amorphous phase, a thermal treatment at 450°C for 30 minutes was carried out in order to crystallize them into anatase.
ZnO coating.— Hydrothermal route
Titania NTs were decorated with ZnO nanowires (NWs) by hydrothermal synthesis. First, the titania nanotube substrates were seeded with a ZnO thin layer by a solution of 10 mM zinc acetate (ZnOAc) in absolute ethanol. The substrate was inserted into an empty round-bottom flask and evacuated for 5 min, then 1 mL of solution was injected into the flask. The system was maintained under vacuum conditions for 30 min and then backfilled with ambient air. The impregnation was repeated five times allowing a homogeneous covering among the inner and external side of the walls. The samples were calcined in air at 350°C for 20 min to remove the organic fraction and promote the crystallization of the ZnO seeds.
In a second step, the seeded substrate was immersed in a growth bath containing 50 mM zinc nitrate hexahydrate (Zn(NO3)2 ·6H2O, purity 98%, Sigma–Aldrich), 25 mM hexamethylenetetramine (HMT, purity 98%, Sigma–Aldrich), 1.5 mM polyethyleneimine (PEI, MW = 800 g mol−1, end capped) and 320 mM ammonium hydroxide in bi-distilled water (from a Direct-Q Millipore purification system) and maintained at 88°C for 2 hours.9 At the end each sample was thoroughly washed with bi-distilled water and dried under nitrogen flow.
Electrodeposition
The decoration of the titania surface with ZnO nano and microparticles (NPs and MPs) was performed by electrodeposition (ED). The remaining Ti foil, presented at the bottom of the titania nanotubes, served as working electrode. The electrodeposition was performed in a two electrodes system with a Pt foil acting as counter electrode. The electrolyte solution used for ZnO deposition consisted of 0.01 M zinc nitrate hexahydrate in bi-distilled water. The ED was carried out at 2.5 V, 85°C for 2 hours and at the end the samples were washed with water and dried with nitrogen.
Reference samples
In order to decouple the nature of the material from the effect introduced by the roughness of the material surfaces in the case of nanostructures, either flat (thin films) and nanostructured (nanotubes and nanowires) TiO2 and ZnO were fabricated and characterized by contact angle measurements.
A thin TiO2 layer was obtained by casting a commercial paste (Ti-NANOXIDE D from Solaronix) onto glass surface according to the recipe previously reported.3
To obtain a flat layer of ZnO, a solution of 10 mM of zinc acetate in ethanol (5 mL) was spin coated on the cleaned silicon wafer at 1000 rpm for 20 s and at 3000 rpm for 30 s. The substrates were then washed for few seconds in ethanol and dried under nitrogen flow. This process was repeated 5 times. Then the substrates were calcined in air at 350 °C for 20 min (heating rate 5°C/min) to obtain a thin and flat layer of crystalline ZnO.9,12
Bare ZnO nanowires were synthesized on a seeded flat substrate (silicon wafer), prepared as described for the flat films of ZnO, from a 10 mM solution of zinc acetate in ethanol and hydrothermally grown using the solution reported above for 2 h at 88 °C.9
Characterization methods
The morphology of the ZnO-decorated samples was characterized by Field Emission Scanning Electron Microscopy (FESEM, ZEISS Dual Beam Auriga) equipped with an Energy Dispersive X-ray detector (EDS, X-MaxN 50 mm2; Oxford Instruments). X-ray diffraction system (XRD from X'Pert) with Cu-Kα X-ray tube (λ = 1.542 Å) with an accelerating voltage of 40 kV was used to characterize the crystalline structures of both ZnO-decorated titania nanotubes and the titania host itself.
The wetting behavior of the samples was evaluated in short time after the sample preparation by optical contact angle measurements by the sessil drop technique with an OCA H200 instrument (DataPhysic Instruments GmbH) in ambient conditions. A DI-water drop with a volume of 1.5 μL was dispensed, and the image of the drop on the sample was acquired with the integrated camera. The drop profile was extracted and fitted with dedicated software that returned the contact angle value at the liquid–solid interface. For each sample, three drops were dispensed at three different positions on the surface and the average value was obtained from the software.
Fourier Transform Infrared Spectroscopy (FTIR) measurements were carried out on a Bruker Tensor 27 in Attenuated Total Reflectance (ATR) mode.
Results and Discussion
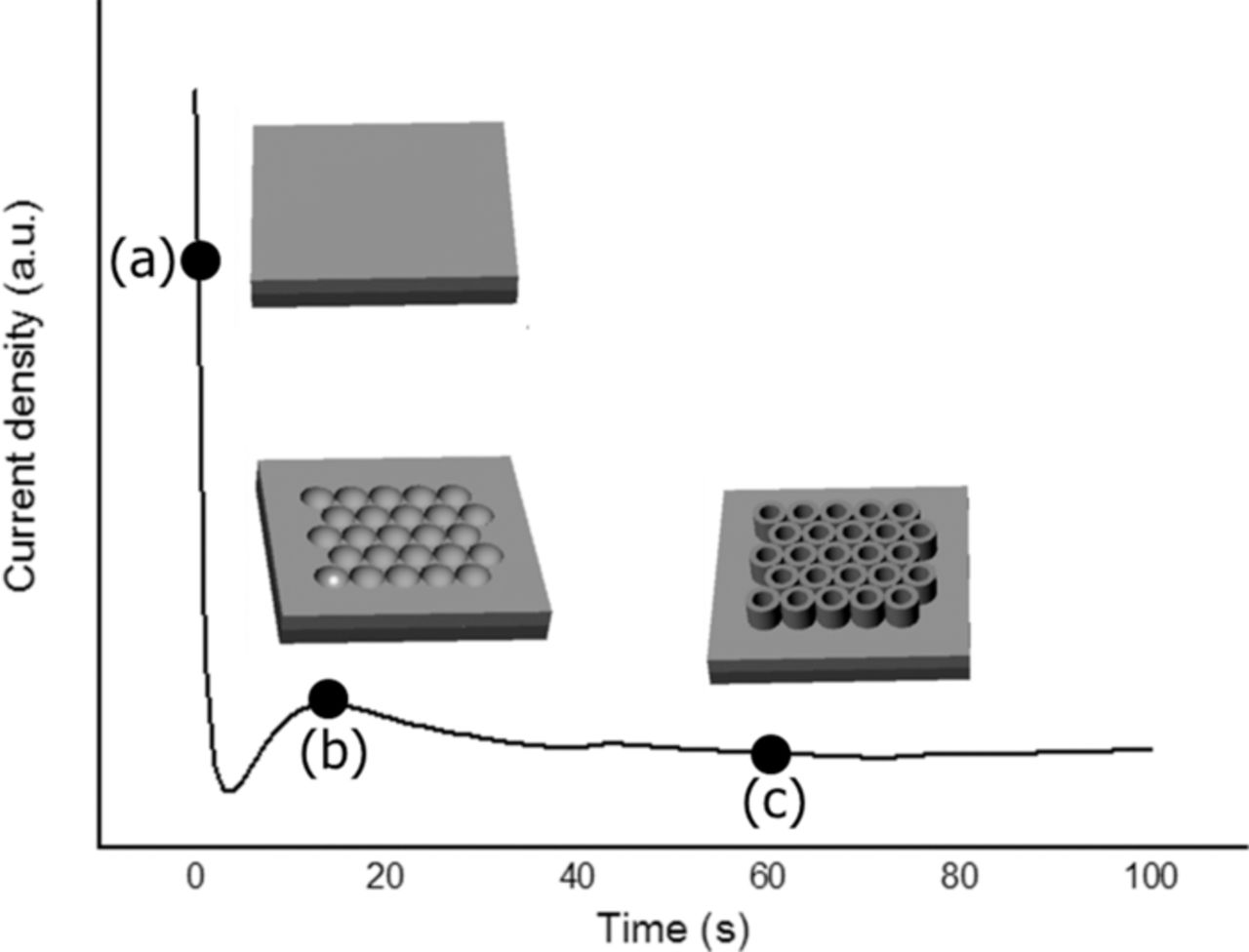
The mechanism of TiO2 nanotube growth in electrolyte solutions containing fluoride ions starts with the oxidation of the metallic surface. The formation of the oxide can then be monitored by recording the current–time characteristics during growth. In Figure 1 a typical current density–time curve for conditions leading to nanotube formation is reported. The curve shows three regimes: in the initial stages of anodization (zone a) in Figure 1) a compact oxide layer is formed. In stage b), a current increase occurs and nanoscale pores are initially formed penetrating the initial compact oxide (the current increases as the reactive area increases). These locally etched pits act as pore forming centers, which subsequently convert into pores uniformly distributed over the whole surface. The pores start to grow at the bottom with inward movement of the oxide layer. In step c), the current slowly drops again while a regular nanotube layer is formed. The final titania nanotubes have a length of 5 μm and an external diameter of about 100 nm.
Figure 1. Schematic representation of anodic oxidation process of Ti into fluorine-based electrolyte matched with the current density versus anodization time characteristics recorded during the process.
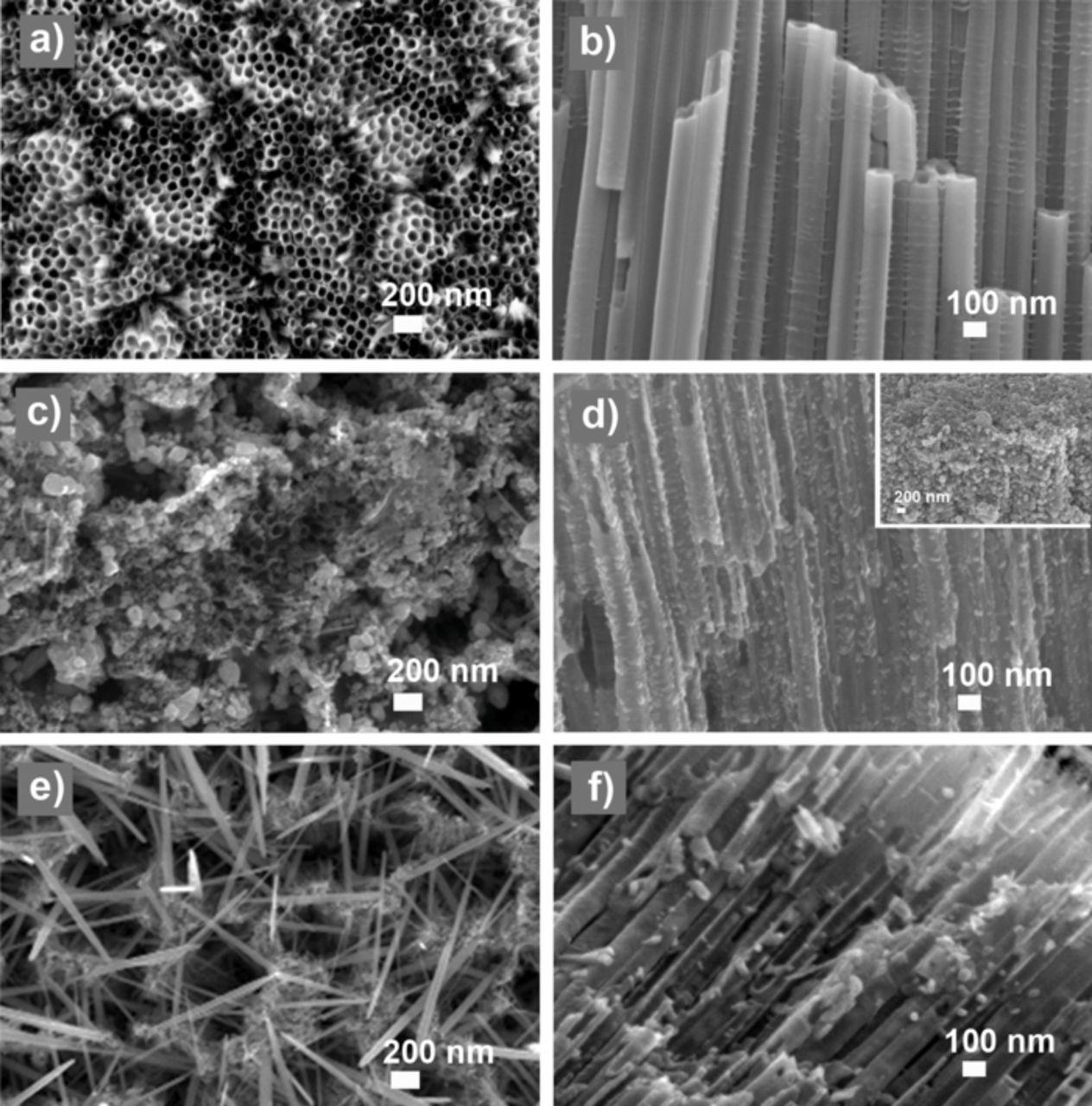
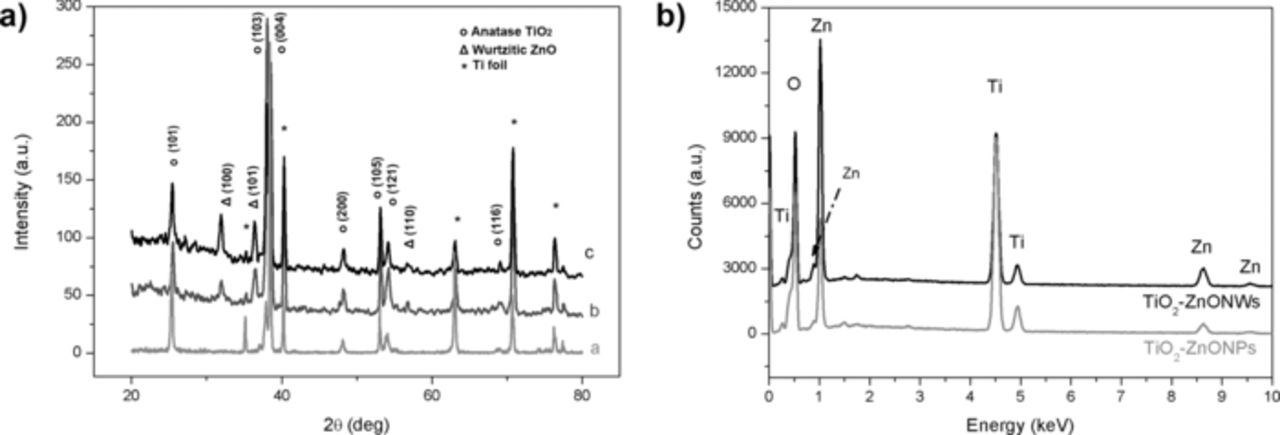
As shown in Figure 2a, the bare nanotubes have an open end at the top. Thus, the internal part of the nanotubes can be easily accessible for the ZnO precursor solutions resulting in the ZnO material deposition. In addition, cross-sectional imaging (Figure 2b) demonstrates the presence of empty spaces among the titania nanotubes where the solution could easily move in. The XRD spectrum (Figure 3a, curve a) of the calcined titania NTs shows the typical pattern of the anatase phase. The observed peaks correspond to the (101), (200), (105), (121) and (116) crystalline planes. In addition, the peaks indicated with the symbol (*) are related to the remaining Ti foil present at the bottom of the TiO2 NTs after the anodic growth. The mean size of the anatase crystallites was estimated through the Debye-Scherrer model, obtaining a value of 33 nm.
Figure 2. FESEM images of titania nanotubes (a-b) as made, (c-d) after electrodeposition, obtaining ZnO nanoparticles and (e-f) after hydrothermal growth, decorated with either ZnO nanowires at the top surface and nanoparticles at the inner lumen. Top view images are presented in the left column, whereas cross-section images are on the right. The inset of Figure 1d shows a 45°-tilted image of the top of the titania tubes.
Figure 3. a) XRD spectrum for the bare titania NTs (curve a), the TiO2-ZnO hierarchical sample by electrodeposition (curve b) and by hydrothermal method (curve c). Figure b) shows the EDS spectra (acquired from the top of the sample) of the titania NTs decorated by ZnO micro- and nano-particles or by ZnO NWs.
After impregnation of the substrates by the ZnO precursor solutions and either hydrothermal or electrodeposition reactions, a regular covering of the titania nanotubes by both ZnO nanoparticles (Figures 2c, 2d and 2f) and nanowires (Figures 2e) is observed, leading to hierarchical nanostructures. The nanoparticles were obtained by the electrodeposition of ZnO, starting from a zinc nitrate solution. This solution is usually used for the synthesis of ZnO films on flat electrode.16 In the present case, the nucleation of the ZnO takes place randomly on the surface of the nanotubes forming a layer of nanoparticles of circa 20 nm in diameter. A homogeneous filling of the titania NTs by the ZnO nanoparticles is observed. On the top of the nanotubes (see inset of Figure 1d), ZnO microparticles (MPs) are formed due to a further merge of the nanoparticles until having diameters as big as 150 nm. We propose the following growth mechanism to explain the difference between the ZnO NPs diameter at the tube inner surface and those (ZnO MPs) at the top of the substrate. At the beginning of the electrodeposition process, the solution containing Zn2+ species can reach all the surfaces exposed by the titania NTs sample. Once the ZnO nanoparticles are formed and decorate the inner surface of the NTs, they grow inwards the NTs center, thus blocking at a certain point the further access for the ZnO precursor solution into the NTs lumen. Thus, only the top of the sample is further accessible for the precursor solution resulting in a covering of ZnO microparticles.
Regarding the decoration of the TiO2 NTs with ZnO nanowires, the hydrothermal approach was used. Here, a difference between the top of the NTs sample (Figure 2e) and the internal wall of the tubes (Figure 2f) can be also observed. On one hand, the openings of the nanotubes at the top side of the sample are covered by a network of ZnO nanowires of about 1 μm in length and a diameter of about 50–80 nm, oriented with small angles with respect to the surface, thus crossing each other and forming a needle-like network. On the other hand, ZnO nanoparticles of about 20 nm in diameter were formed at the inner NT walls, showing a good filling ratio. These results demonstrate that the growth of the nanowires was possible only at the top surface, where more reagents were available. As similarly explained before, the formation of ZnO material blocks the further growth of the nanostructures within the titania nanotubes, preventing the access of the precursor solution and thus the formation of ZnO NWs inside the titania NTs.
There are no major differences between the XRD spectra of the hierarchical nanostructures, i.e. titania NTs covered by either ZnO-NPs (Figure 3a, curve b) or the ZnO-NWs (Figure 3a, curve c). The two methods of ZnO coverage studied in this work formed wurtzite-type crystal structure ZnO nanostructures, confirmed by the presence of the (100), (101) and (110) reflections observed at 31.9°, 36.4° and 56.8°, respectively, in the XRD patterns. A slightly higher level of crystallinity can be observed in the TiO2-ZnO hierarchical sample obtained by hydrothermal method, since the ZnO NWs are generally single crystalline structures, whereas ZnO nanoparticles are polycrystalline.9,11 The main dimension of the crystalline domains is equal to 21 nm for both samples, as evaluated through the Debye-Scherrer equation. This value demonstrates that the nanoparticles inside the TiO2 NTs are single crystalline in both samples. The (101) reflection of anatase still remain visible in both spectra of the TiO2-ZnO hierarchical samples, excluding any possible deterioration of the titania crystalline structure by the further ZnO growth reactions. In particular, the anatase crystallite size remained the same. The EDS measurements performed on the top surface and in the cross section of the hierarchical samples confirm the presence of Zn within and over the titania NTs (see patterns in Figure 3b).
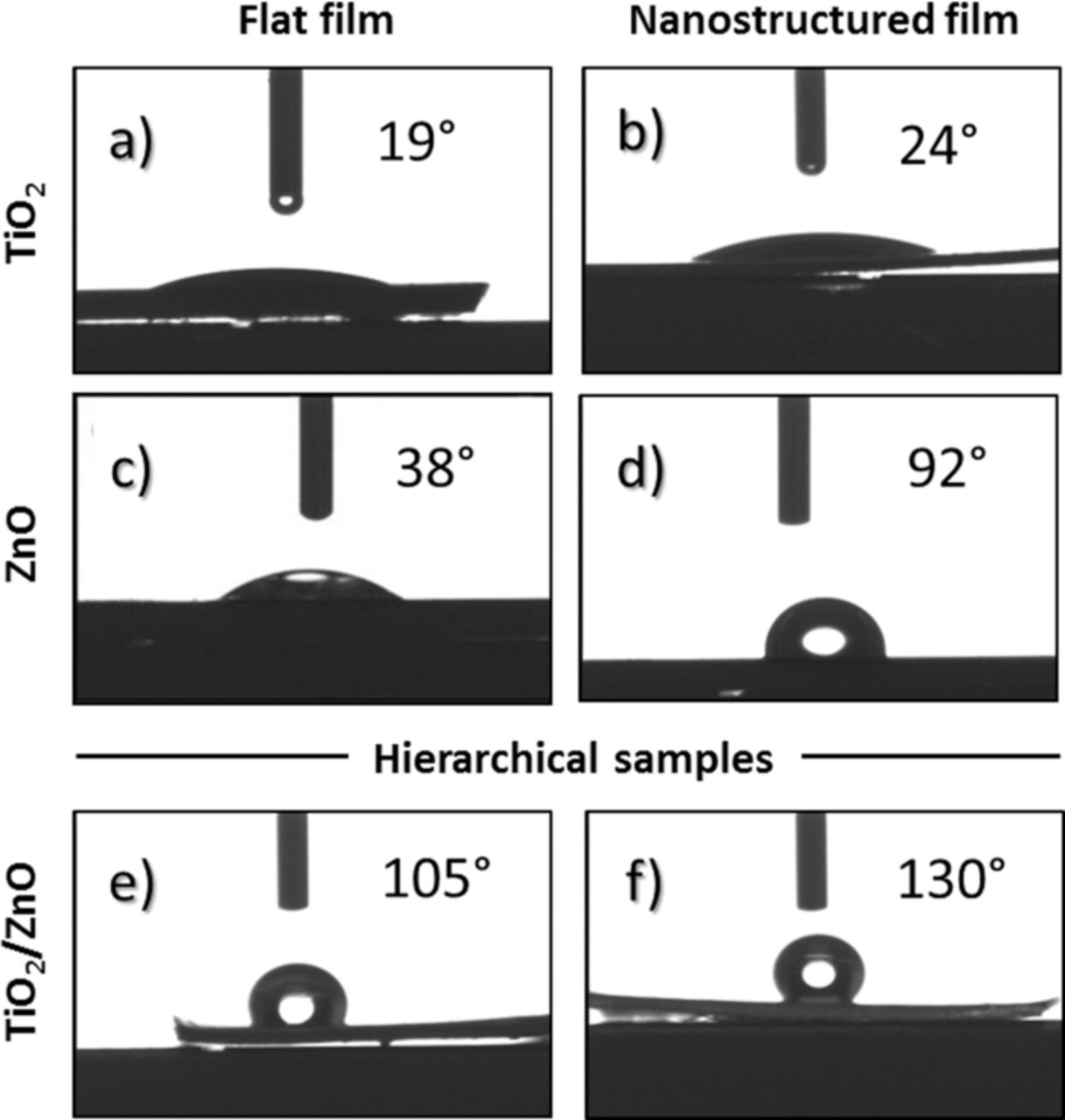
In order to evaluate the surface properties of our hierarchical nanostructured materials, water contact angle (WCA) measurement were carried out. In this case two main contributions can affect the WCA behavior, i.e the composition of the material and its roughness introduced by the nanostructuration. In order to decouple these two effects, flat (thin films) and nanostructured (nanotubes and nanowires) TiO2 and ZnO materials were opportunely synthesized. Figures 4a and 4c show the contact angles evaluated on both TiO2 and ZnO flat surfaces, respectively. They both exhibit a high water wettability, as previously reported in the literature.31,32 By modifying the morphology of the material at the nanoscale, the water contact angles vary from 19° to 24° for the TiO2 nanotubes and from 38° to 92° for the ZnO nanowires (see Figures 4b and 4c). Similar results were obtained by Balaur et al.33 who demonstrated that tubular TiO2 films were hydrophilic in the as-processed state, as well as ZnO nanowires synthesized hydrothermally on a sol-gel deposited seed layer,12 as in the present case.
Figure 4. Water contact angle on a) flat titania film, b) bare titania NTs, c) flat ZnO film, d) bare ZnO nanowires synthesized by hydrothermal method, hierarchical e) TiO2-ZnO MPs, and f) TiO2-ZnO NWs.
After the deposition of ZnO nanostructures by electrodeposition, we have shown the formation of microparticles of about 150 nm at the top surface of the titania NTs. The WCA is greatly increased (WCA = 105°) resulting in a hydrophobic surface (generally when WCA > 90°). After the hydrothermal process, when ZnO NWs were formed on the TiO2 NTs top surface, the hydrophobicity is even higher, resulting in WCA of 130° which is almost superhydrophobic.
It is well known that WCA of a surface depends strongly on the chemical composition (i.e. material dependent surface energy), as well as on the morphology of the outermost surface (surface roughness, presence of micro and nanostructures).34–36 Our hierarchical TiO2-ZnO nanostructures show a dramatic change of the surface wettability and we attribute this modification to different contributions, as explained below, due to the formation of a network of ZnO nanostructures at the top surface of the titania NTs. The phenomenon of surface wetting behavior is well understood and explained by either Wenzel35 and the Cassie–Baxter37,38 models. In the Wenzel state, it is assumed that the liquid drop completely wets all the topographical features of the surface and only the solid and liquid interfaces are considered. In the Cassie model,34 the applied liquid is presumed to not penetrate into the surface features, thus lying on top of the topographical facets. This effect is also referred as the "Fakir" state.39 Under this regime solid, liquid and air interfaces are considered. Depending on the surface structure and material composing the surface, transitions between these two models are possible.37 Some of us have previously reported12 that the wetting behavior of ZnO is strongly dependent on the density of lattice defects: the lower the number of oxygen vacancies, the lower the number of hydroxyl groups (-OH) on the oxide surface and thus higher the overall hydrophobicity.
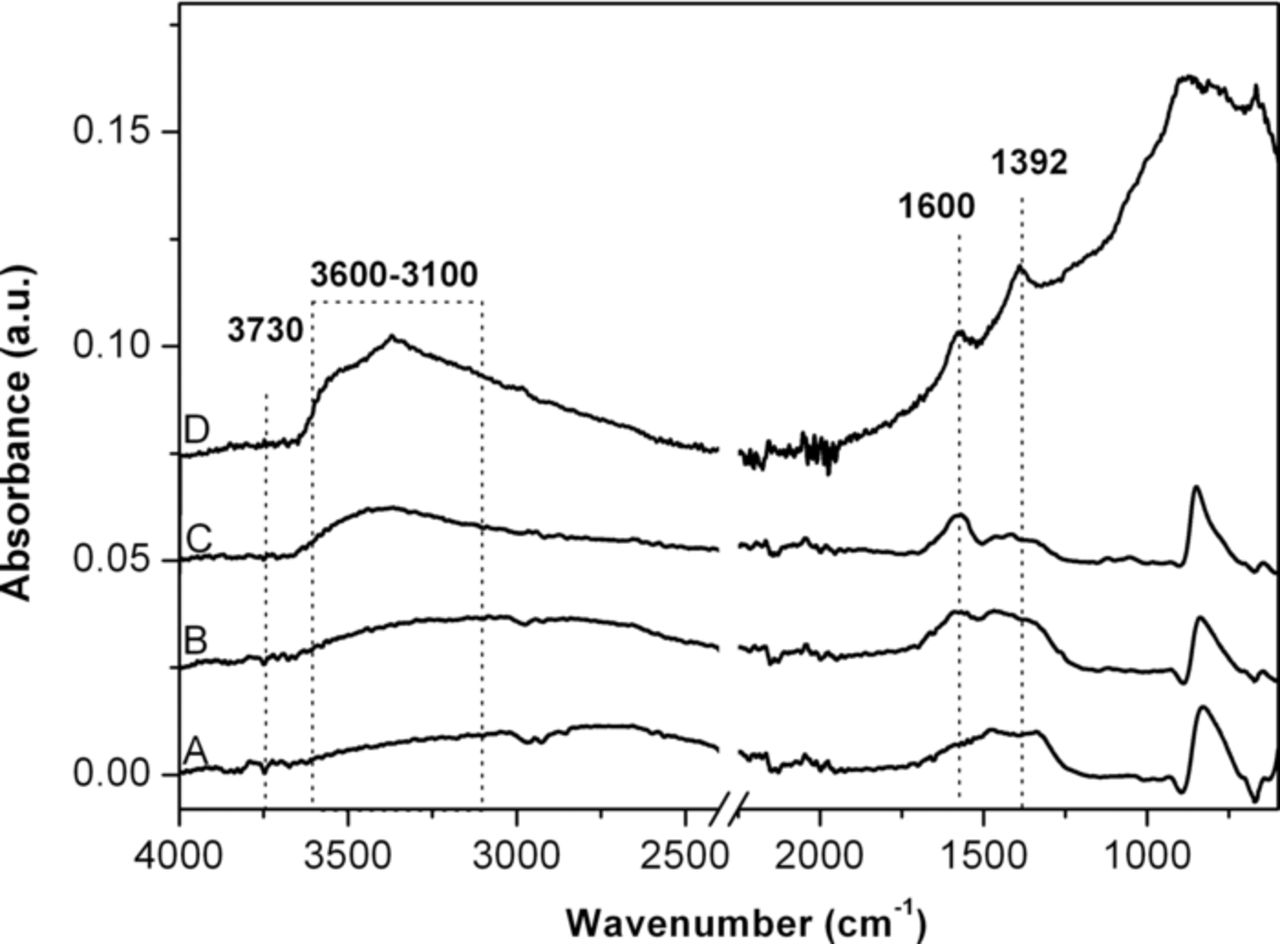
To confirm this assumption, we have carried out FTIR measurements, as presented in Figure 5. The surface of the bare ZnO NWs (curve A) is rich of hydroxyl groups. In particular at 3730 cm−1 isolated surface hydroxyl groups are observed. The broad band in the range 3600–3100 cm−1 is attributed to OH stretching from the water of crystallization in solid state, and that at 1600 cm−1 to the OH deformation. Indeed the WCA measurement on this sample still indicate some level of hydrophilicity (WCA = 92°). In contrast, both hierarchical nanostructured samples show a reduced intensity of the above mentioned vibration peaks (curves B and C), resulting in an increased level of hydrophobicity. In particular the surface of TiO2-ZnO material made by hydrothermal method does not present any evidence of hydroxyl groups (curve C) resulting in a pronounced hydrophobicity with WCA of 130°. We can assume that the ZnO NWs on the surface of the hierarchical sample synthesized by hydrothermal route are less defective than the ZnO microparticles obtained by the electrodeposition method on the top surface of the TiO2 nanotubes. ZnO NWs are generally single crystalline nanostructures having an hexagonal wurtzite phase and anisotropic growth along the (001) axis, i.e. the c-axis.40 In contrast the ZnO microparticles of 150 nm in diameter are formed as a random aggregation of single crystalline nuclei, without any preferential orientation, thus more defects and oxygen vacancies are expected in the overall nanostructure.
Figure 5. Fourier Transform Infrared (FTIR) spectra in Attenuated Total Reflectance (ATR) of the bare ZnO nanowires (Curve A), hierarchical TiO2-ZnO sample obtained by electrodeposition (curve B) or hydrothermal method (curve C), and bare titania NTs (curve D).
The particle morphology also plays a fundamental role on the wetting behavior: here the hierarchical TiO2-ZnO surface characterized by a network of sharp nanowires is indeed highly rough and nanostructured, more than the surface with deposited ZnO nanoparticles. Similarly, the bare ZnO NWs have shown a higher WCA with respect to the flat ZnO surface. Rough surfaces are indeed able to easily trap air pockets thus enhancing the surface hydrophobicity. In our case it is clear that the portion of trapped air is higher on a surface composed by ZnO nanowires than one of microparticles, in both bare and hierarchical samples.
As a result of all these contributions, i.e. the reduced presence of oxygen vacancies, absence of hydroxyl groups, higher crystallinity, higher level of nanostructuration and trapped air pockets, both hierarchical TiO2-ZnO samples are highly hydrophobic with respect to the bare TiO2 NTs or ZnO NWs. In addition, the hierarchical TiO2-ZnO NWs sample shows a higher hydrophobicity with respect to the hierarchical TiO2-ZnO NPs one.
Conclusions
We have reported on the anodic oxidation of titanium foils to obtain a regular array of vertical titania nanotubes of about 5 μm in length and 100 nm in diameter. The nanotubes have shown a crystalline anatase phase after proper thermal treatment and high hydrophilicity. We have then decorated the inner and outer walls of the NTs with ZnO nanoparticles, of about 20 nm in diameter, using two different synthetic approaches: the electrodeposition and the hydrothermal methods. In this way hierarchical TiO2-ZnO nanostructures were obtained showing on the top surface either ZnO microparticles of 150 nm, when using electrodeposition, or ZnO nanowires of 1 μm in length, when adopting the hydrothermal route as synthesis method. Both hierarchical TiO2-ZnO samples have shown the formation of wurtzite-type crystal structure ZnO nanostructures and a dramatic change of the wetting behavior of the top surface toward hydrophobicity. In particular, hierarchical TiO2-ZnO NWs are more hydrophobic (WCA = 130°) than the hierarchical TiO2-ZnO NPs (WCA = 105°). We have proposed that several factors are responsible for this wettability change, such as the presence of structural defects and oxygen vacancies, reduced presence or even absence of hydroxyl groups, as well as the level of nanostructuration of the top surface of the samples.
These hierarchical TiO2-ZnO samples can find potential applications in anti-fogging, anti-reflecting and self-cleaning surfaces for photocatalytic devices, as well as biomedical hard tissue implants.