Abstract
Stable, reproducible, and well-defined reference electrodes are vital for accurate quantification of battery material properties and physical mechanisms. Repeated studies have shown sodium metal to be an unreliable reference electrode material in nonaqueous solvents. This work evaluates several alternative reference electrode chemistries including sodium-tin alloy, nickel hexacyanoferrate and carbon. Of the systems tested, non-aqueous silver/silver-ion reference electrodes were shown to yield the most stable and reproducible voltage measurements.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
In recent years, interest in sodium-ion batteries as a low cost, earth-abundant alternative to lithium-ion batteries for grid scale and other stationary energy storage applications has increased.1 While important strides are being made to increase the storage capacity and lifetime of sodium ion batteries, the successful adoption of sodium batteries depends on identifying affordable, high-performance, long-lasting electrodes and electrolytes. These advances rely on careful measurements of electrode and electrolyte phenomena. Accurate measurement of rate capability, charge capacity, and lifetime is necessary to assess the value of a given material, while understanding of storage and degradation mechanisms allows better design of materials with increased performance.
Many battery electrode studies utilize a two-electrode configuration, in which the counter electrode acts as both the current source/sink and the reference potential against which voltage at the working electrode is measured. Two electrode tests can be configured as either a "full-cell", where the electrode of interest is tested against lithium or sodium metal, or a "full-cell", where both the anode and cathode are materials of interest. Two electrode experiments benefit from experimental simplicity and ease of construction, but convolute the behavior of two materials. Slow reactions at the counter electrode generate overpotential that contributes to the measured voltage, and larger distances between the working and counter can increase the influence of ohmic loss in solution. These effects make it difficult to differentiate between processes at the individual electrodes. Increasing polarization at the counter electrode can even give the erroneous appearance of a phase change at the working electrode,2 or artificially limit the voltage window of the working electrode.3,4
The addition of a reference electrode, i.e. a three-electrode set-up, addresses these concerns by separating current from voltage measurement. The counter electrode still supplies the necessary electrons, while voltage is measured against the third electrode, termed the reference. Three-electrode configurations also allow independent measurement of anode and cathode voltages in full-cell configurations. The ideal reference electrode should be non-polarizable and have a constant composition over time in order to provide a stable, reproducible potential.
Sodium metal is currently the standard material for reference electrodes in studying sodium-ion systems, but it has been shown to be unstable and poorly reproducible. Instability of the SEI on sodium metal has been shown to cause impedance increase and unreliable voltage measurements when using sodium metal as a reference.5,6 Furthermore, we have recently shown that sodium metal reference electrodes can form soluble electroactive degradation products which can result in erroneous interpretation of electrode behavior.7 It is therefore of upmost importance to develop reliable reference electrodes for accurate three-electrode measurements in nonaqueous sodium-ion systems.
While the majority of sodium-ion studies have used sodium metal as a reference electrode, some groups have used pseudo-reference electrodes to measure behavior in sodium-ion systems. Silver wire has been used as a pseudo-reference for a range of alkali cations including sodium.8 More recently, activated carbon was used as a pseudo-reference to explore the effects of sodium metal on cycling stability.9 Because the reactions that govern a pseudo-reference potential are inherently ill-defined, these electrodes are often strongly influenced by surface impurities and electrolyte contaminants. In contrast, a well-defined reference electrode offers a consistent standard across different electrolyte systems to provide a more reliable measurement. To the best of our knowledge, a well-defined reference electrode for non-aqueous sodium-ion batteries has not been published. Alternative reference electrodes have been explored more extensively in lithium-ion systems. Metal alloy electrodes, including tin, gold, bismuth and aluminum, have been demonstrated in lithium systems.10,11 Insertion electrode materials, such as lithium titanate and lithium iron phosphate, have also been used as reference electrodes.12,13 In this work, we investigate the use of alloying and intercalation electrodes as alternatives to sodium metal, in addition to a carbon pseudo-reference and commercially available silver ion electrode.
Experimental
All chemicals were from Sigma Aldrich (USA) unless otherwise specified.
Nickel hexacyanoferrate formulation
Nickel hexacyanoferrate (NiHCF) was made by the dropwise addition of 0.5 M nickel sulfate and 0.5 M potassium hexacyanoferrate in a 2:1 ratio into a continuously stirred beaker of DI water. The solution was allowed to sit overnight and the precipitate was triple rinsed and centrifuged. Material was dried for 24 hours in a vacuum oven to remove excess water.
Electrodes were prepared using NiHCF, carbon black (CB) (MTI, Super C65), and polyvinylidene difluoride (PVDF) (Arkema, MW = 380k) in a 75:15:10 ratio in N-Methyl-2-Pyrrolidone (NMP). The slurry was coated onto an aluminum foil current collector using a bird applicator at 100μm. Carbon composite electrodes were made with a 90:10 CB:PVDF in NMP on a copper foil current collector. Electrodes were dried at room temperature for approximately 2 hours, then at 110°C overnight in a vacuum oven.
As-fabricated NiHCF electrodes were characterized through cyclic voltammetry in 1M sodium sulfate using a platinum counter electrode and silver-silver chloride reference electrode (3M NaCl, BASI, Inc.).
Tin alloy
A tin-sodium alloy reference electrode was produced by galvanostatically intercalating sodium in a 0.5 cm long section of a 0.5mm diameter tin wire (99.9985%, Alfa Aesar) in 1M sodium perchlorate with a sodium metal counter and reference electrode.
Electrochemical setup
Non-aqueous characterization of candidate reference electrodes was carried out in 1M sodium perchlorate in propylene carbonate (PC), with or without 2 mM ferrocene. Measurements were taken at a glassy carbon working electrode with sodium metal or platinum wire as the counter electrode. Both the counter and reference electrode were placed in individual fine porosity glass frits (Chemglass). Sodium electrodes were made by wrapping a small amount of polished sodium metal (99.9%, Sigma Aldrich) around the stripped end of an insulated copper wire. Commercial silver ion electrode (Basi, Inc.) had a filling solution of 10 mM silver nitrate in PC with 100 mM tertrabutyl ammonium perchlorate (TBAP) as supporting electrolyte salt.
Characterization
Hexacyanoferrate electrodes were characterized with X-ray diffraction (XRD) (Rigaku Smartlab) using Cu K-α radiation at 40 kV and 30 mA for 2theta between 20 and 80.
The concentration of silver ions in solution was measured using flame atomic adsorption spectroscopy (flame AA) (Varian AA240FS), at 328.1nm and slit width of 0.5nm with an air-acetylene flame.
Testing protocol
Potential electrode materials were evaluated on the basis of voltage stability and reproducibility over hours and days, as detailed in Figure 1. Short term stability was evaluated at open circuit (OCP) in sodium perchlorate electrolyte without a redox mediator present. Voltage was measured against either an identical candidate reference electrode or known reference. Electrodes with a high degree of noise or rapid change in potential after initial equilibration, approximately 1 hour, were eliminated from further consideration. Subsequently, the potential of ferrocene redox couple was measured versus at least two reference electrodes for each candidate material to evaluate reproducibility. Materials that exhibited acceptable reproducibility were then tested for stability. Repeated cyclic voltammograms were taken in increments of 20–60 minutes to evaluate the drift in the reference electrode potential with time.
Figure 1. Testing protocol for candidate reference electrodes.
Reference electrodes that satisfied the initial requirements for reproducibility and stability were then used to measure SEI formation and cycle performance via cyclic voltammetry (CV) with a separate glassy carbon working electrode cell. The ferrocene redox potential was compared before and after cycling to evaluate changes in the reference electrode after exposure to electrolyte degradation products in solution. A drift rate of 0.4mV/hr or a step change of 10mV following cycling was considered unacceptable, as 10 mV error could shift reactions from insertion to metal plating at the working electrode. The performance of candidate materials during steps II and III is summarized in Table I.
Table I. Performance metrics for candidate reference electrodes.
| Standard deviation in initial E1/2 (mV) | Drift in Ferrocene E1/2 (mV/hr) | Δ E1/2 After Cycling (mV) | |
|---|---|---|---|
| Ag Ion | 1.5 | 0.21 ± 0.01 | 3.0 |
| NiHCF Composite | 36 | 3.2 ± 1.7 | 19.5 |
| Carbon | 5 | 5.1 ± 4.8 | 236.0 |
Results and Discussion
Tin alloy
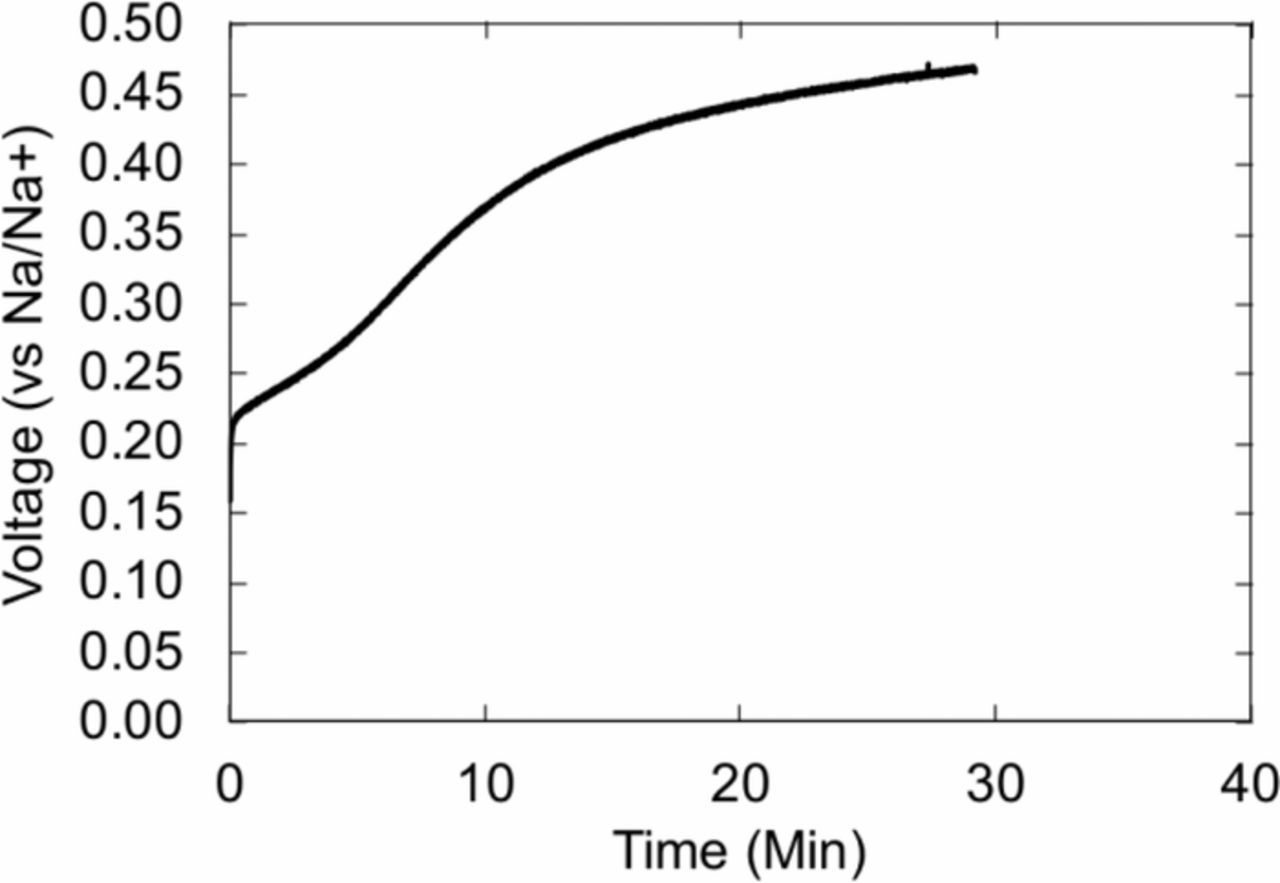
While Abraham et al. have demonstrated success with lithium-tin alloy electrodes,10 sodium-tin alloy was found to exhibit severe potential drift. Figure 2 shows the OCP in 1M sodium perchlorate immediately after electrochemical alloying. This increase in potential can be attributed to self-discharge, or electrochemical dealloying, coupled to spontaneous electrolyte reduction. In contrast to lithium-ion systems, sodium-ion systems are known to form highly soluble SEI layers. Thus, reference electrodes with reactions below the electrolyte stability window are subject to self-discharge through electrolyte reduction.14,15 Pre-formation of an artificial SEI may provide a stable protective layer to prevent SEI degradation.16 Alternatively, electrodes that operate at equilibrium potentials, greater than approximately 1 V vs Na/Na+, may be more successful at preventing electrolyte reduction and subsequent electrode drift. Antimony has the highest reported voltage for sodium alloys, with average voltages for SbNa around 0.75 V.17 Other candidate electrode materials include metal oxide conversion electrodes, including SnO2, Sb2O4, CuO, Fe3O4, with average voltages in the range of 1−1.5V, and NASCION-type Na3Ti2(PO4)3 with a plateau at 2.1V.18 However, the use of intercalation electrodes as references results in different complications, as discussed below.
Figure 2. Behavior of NaxSn electrode at open circuit in 1M NaClO4 vs sodium metal reference electrode.
Insertion materials
Prussian blue analogues have been studied as potential cathode materials for lithium- and sodium-ion batteries.19,20 We investigated the reference electrode behavior of nickel hexacyanoferrate, KNiFe(CN)6, based on its open structure, flat voltage profile and fast electron transfer reference electrodes. Multiple methods were tested to produce hexacyanoferrate structures, including electrodeposition and electroless dip coating of nickel wires. Only particle-based electrodes are discussed here. The synthesis of particles and fabrication of a composite electrode cast from a slurry yielded the most consistent reproducibility of voltages and best stability. The NiHCF structure was validated using X-ray diffraction, shown in Figure 3a. Major peaks at 17.28, 24.52, 35.04 and 39.40, corresponding to the (200), (220), (400) and (420) phases, with additional peaks at 43.24, 50.6, 53.8, 57, 65.8, and 68.56 corresponding to the (422), (440), (600), (620, (640) and (642) phase respectively, show good agreement to structures synthesized by other groups.21
Figure 3. Characterization of Nickel Hexacyanoferrate (NiHCF) composite electrode with (a) XRD and (b) CV in 1M sodium sulfate for four replicates of the NiHCF composite electrode demonstrating reasonable reproducibility of electrode potential, as measured by half-wave potential, E1/2.
Voltammetric characterization of NiHCF composite electrodes in 1M sodium sulfate is shown in Figure 3b. The redox potential for reversible sodium insertion into the lattice occurs at 405 +/− 1.2 mV, demonstrating a stable structure with reproducible potential. Electrodes were not optimized for rapid charge transfer, so variation in peak splitting is likely the result of differences in mixing and material distribution.
While the composite electrodes showed the best stability, drift rates between 2 to 10 mV/hr were observed. The measured redox potential of the ferrocene-ferrocenium couple drifted from approx. −300 mV vs the reference electrode to approx. +50 mV. The characteristic drift in ferrocene redox potential versus time is shown in Figure 4a. A self-discharge that coupled NiHCF reduction to ferrocene oxidation is one possible source of this drift. However, the reference electrode continued to drift after reaching potentials lower than the ferrocene reaction, the electrode drifted during experiments in neat electrolyte without ferrocene, and pre-soaking in ferrocene reduced but did not eliminate the initial drift. Therefore, reduction of NiHCF by reaction with other electroactive products is necessary to fully account for the change in potential. Because sodium metal can produce electroactive degradation products that undergo oxidation at voltages as low as 1V7 the sodium metal counter electrode was replaced by a platinum wire to limit the influence of degradation products from the counter electrode. However, formation of electroactive degradation products at a platinum electrode is also possible.
Figure 4. (a) Stability of potential for ferrocene/ferrocenium reaction versus selected reference electrodes and (b) ferrocene CVs before and after reference electrodes were used in SEI characterization study. Reference electrodes are nickel hexacyanoferrate (blue), carbon (green) and silver ion (orange).
Activated carbon
Activated carbon electrodes have been demonstrated as a pseudo-reference electrodes in non-aqueous lithium.22 More recently activated carbon reference electrodes were used to evaluate the impact of the sodium metal electrode on measured battery capacity.9 The reference electrode voltage stability was not directly studied, but some influence of carbon surface chemistry was observed.
We evaluated carbon black composite electrodes as well as graphite rods as reference electrodes. The ferrocene oxidation was initially observed at 110 +/− 5 mV and the potential drifted by between 4–10mV/hr, as shown in Figure 4a. The carbon electrode potential is highly sensitive to surface functionalization. Therefore, this drift can be tentatively attributed to anion adsorption or reactions between electrolyte and carbon surface groups. The reactivity of the carbon pseudo-reference with electrolyte components is further supported by the change in potential after use. After the carbon pseudo-reference was used to cycle a glassy carbon working electrode (Figure 1 step III-B), the ferrocene redox potential increased by 236mV, as shown in Figure 4b. This change is not accounted for by the baseline drift in electrolyte and shows that solution changes during cycling, particularly the formation of soluble electrolyte degradation products can cause changes in the carbon reference potential. In pseudo-reference electrodes, the redox couple that defines thermodynamic equilibrium for electrons is not well-defined, so stable measurements depend on the electrode potential and bulk solution remaining unchanged during testing. The success of carbon pseudo-reference electrodes depends on a high surface area to limit the effect of surface adsorption on measured potential. The use of larger reference electrodes or activated carbon can provide a greater internal surface area to slow the drift rate, but drift will likely always occur.
Silver ion
Silver ion reference electrodes are widely used in non-aqueous potentiometry and commercially available.23 Silver ion reference electrodes utilize an Ag/Ag+ redox system consisting of silver wire in a dilute solution of silver ions. In contrast to the aqueous Ag/AgCl reference electrode, the Ag+ remains dissolved in solution and must be supplied by dissolving silver nitrate in the same solvent as the system of study. A porous glass frit is used to create a low resistance junction while minimizing cross-contamination. The commercial silver ion electrode had a drift rates of approximately 0.2 mV/hr. This observed drift is in good agreement for the previously reported value of 0.16mV/hr for the same electrolyte in acetonitrile.24 The sensitivity of electrode potential to silver ion concentration and silver contamination was interrogated. The potential shows a Nernstian dependence on the concentration of silver ions, as shown in Figure 5a, and drift rate was independent of silver ion concentration, as shown in Figure 5b. Furthermore, the potential was not dependent on the concentration of sodium ions inside the fill solution, seen in Figure 5c, meaning that diffusion of sodium ions into the frit was not a significant contributor to drift.
Figure 5. (a) Potential of ferrocene reaction as a function of concentration of silver ions, (b) silver ion electrode drift rate for 5 and 10mM fill solution and (c) potential of silver ion with 10mM AgNO3 as a function of added sodium ions.
The impact of the silver ion electrode on the sodium system was investigated by measuring the transport of silver ions into the test solution. The silver ion electrodes were soaked in propylene carbonate for 18 and 72 hours. As shown in Figure 6a, the concentration of silver ions in solution measured by flame AA was 0.2 μg/L after 72 hours, corresponding to a diffusion rate of approximately 3.8E-8 μmol/hr. The introduction of a double junction also reduced this rate even further, bringing the silver in solution after 72 hours to less than 0.015 μg/L. To determine the influence of trace silver on electrolyte reduction chemistry, glassy carbon electrodes were cycled to SEI formation potentials in both neat electrolyte and electrolyte with 2 μM silver nitrate added. The CVs are shown in Figure 6b. No impact on SEI formation behavior or degree of passivation was observed when silver was present, suggesting that the silver ion reference electrode can be used without influencing the behavior of the system. While any silver ions present should be reduced at these system potentials, the low concentration means any silver plating on the surface represents a small fraction, less than 0.5%, of the total electrode area. Silver is generally known as a catalytically inactive metal. It has also been shown electrochemically to be relatively inactive in lithium electrolytes.25
Figure 6. (a) Concentration of silver ions in solution when electrode is soaked directly in solution (solid) and in a double junction (hollow). (b) SEI formation on a glassy carbon electrode with neat electrolyte (orange) and 2uM silver added(blue), inset with same units.
Conclusions
Several candidate materials were evaluated as reference electrodes for accurate measurements in non-aqueous sodium electrolytes. Tin alloying electrodes exhibit significant voltage drift due to spontaneous electrolyte reduction coupled with oxidative self-discharge of the electrode. Considering the insufficient passivation of the sodium-ion SEI, this is likely a problem for all reference electrodes that operate below the cathodic stability window of the electrolyte, approximately 1V vs Na/Na+. Simultaneously, intercalation materials such as hexacyanoferrate compounds that operate at higher potentials are subject to self-discharge. This is likely because sodium-based electrolytes can spontaneously form electroactive degradation products that are oxidized as low as 1V vs Na/Na+. Pseudocapacitive carbon reference electrodes have limited reproducibility and are dependent on high surface area to slow the rate of voltage drift. They also showed a disproportionate voltage change after use as a reference electrode. This change was likely caused by adsorption of or reaction with soluble electrolyte degradation products; therefore the success of carbon reference electrodes will likely vary strongly by system.
Together, insufficient passivation at low potential and oxidation of electroactive degradation products at higher potential yield a very narrow window, if any, for stable reference electrode chemistries. The solubility of electrolyte degradation products in nonaqueous sodium electrolytes ultimately makes any reference electrode in contact with the bulk electrolyte vulnerable to self-discharge. Preventing self-discharge and the associated electrode drift requires either a large capacity of the reference electrode to slow the rate of drift or a physical barrier to isolate the reference electrode from degradation products.
As a reasonable alternative to sodium metal, silver-ion reference electrodes offer a stable and reproducible potential with minimal drift and very little diffusion between the silver and sodium electrolytes. A frit or double junction separates the bulk from the reference electrolyte and helps prolong the useable lifetime of the electrode. We show that crossover of sodium ions does not impact the potential or stability of the silver ion electrode, and that low levels of contamination from silver ions do not impact voltammetry at a carbon working electrode. The implementation of alternative reference electrodes will ensure more reliable electrochemical measurements and better understanding of materials and mechanisms for sodium-ion batteries.
Acknowledgments
Authors thank Dr. Jonathan Soffer for assistance with Flame AA measurements, James L Lansing for performing XRD measurements at the Drexel Central Research Facilities and Thomas J. Heiser for work with electrode fabrication. This material is based upon work supported by the National Science Foundation under grant number 1607991 and the NSF Graduate Research Fellowship Program [SEL].
ORCID
Maureen H. Tang 0000-0003-0037-4814