Abstract
The unique polarization behavior of LiFePO4 electrodes was investigated using mathematical simulations with a many-particle model, which have a non-monotonic potential profile for each LiFePO4 particle, and analyzed by the active population concept. The present simulations reveal two known polarization behaviors, namely the memory effect and path dependence. Notably, a hitherto unknown polarization behavior, the so-called relaxation-induced polarization (RIP), was also identified. The memory effect requires, at a minimum, a sequential four-step operation. By comparison, RIP is triggered only by a one-step operation, namely a long rest. In effect, the polarization associated with the memory effect and RIP was caused by a reduction of the active particles in the two-phase region via relaxation during a rest. The path-dependence mechanism was attributed to kinetically inhomogeneous reactions for each particle. We further found that narrowing the particle size distribution was an effective means to reduce polarization viz. the memory effect and path dependence of LiFePO4 electrodes. However, RIP could not be suppressed by narrowing the particle size distribution. A comprehensive understanding of these polarization behaviors with our model provides a more accurate way to estimate the state of charge in Li-ion batteries with LiFePO4 electrodes.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
As a promising cathode material, LiFePO4 has been commercialized in Li-ion batteries for power tools and electric vehicles because of its low cost, high power, and thermal stability. Unlike other current commercial cathode materials, LiFePO4 undergoes a two-phase reaction between a Li-rich phase and a Li-poor phase with a flat potential profile during charging and discharging.1 It also shows two sloping potential regions at each limit in the Li composition (LixFePO4, 0 ≤ x ≤ 1) corresponding to the solid-solution reaction regions.2,3
LiFePO4 has been reported to exhibit a number of unique polarization behaviors, including voltage hysteresis,4 path dependence,5 and memory effect.6 Voltage hysteresis refers to the phenomenon wherein the charge and discharge potentials of a LiFePO4 electrode do not collapse even at an extremely low rate (C/1000).4 Path dependence refers to a change of polarization in the charge/discharge profiles depending on whether the previous process is charging or discharging.5 Finally, memory effect refers to an unusual polarization bump during charge/discharge, at a state of charge (SOC) where a partial charge/discharge had been terminated previously.6 This effect has been known to be an essential issue for Ni-metal hydride (Ni-MH) batteries7,8 and believed to be absent in Li-ion batteries.
Several mathematical models have been developed to account for these unique phenomena in the electrochemical behavior of LiFePO4, including the shrinking-core model,5 the resistive-reactant model,9 and the many-particle model.4,10 Among all these models, the many-particle model proposed by Dreyer et al.4 shows behavior that is consistent with all the phenomena described above.10 The many-particle model is characterized by a non-monotonic open-circuit potential (OCP) profile within a single particle of LiFePO4, with a sloped (i.e., non-flat) profile in the two-phase region which is opposite to the slopes in the solid-solution regions as a function of Li composition.4,11,12 Such a unique profile has been attributed to the effect of coherency strain on the free energy for two-phase coexistence in the nanoparticles.11 The model also accounts for interactions between particles within the electrode. Dreyer et al. reported that the equilibrium potential of a many-particle electrode during electrochemical processes differed from that of a single particle because of the particle-particle interactions, and explained the hysteresis behavior of LiFePO4.4
Farkhondeh et al.10 incorporated the non-monotonic potential profile and the many-particle concept from Dreyer et al.4 into a mesoscopic model that included the kinetic properties. This model could qualitatively describe the hysteresis, path dependence, and the memory effect. In particular, those authors developed a deep understanding of the memory effect as being caused by the unusual Li composition distribution in multiple particles during the memory-writing cycle. While their results show behavior consistent with the memory effect, there was no detailed explanation of the underlying mechanism. In particular, in the many-particle model, the particles are (dis)charged in a particle-by-particle fashion because of the non-monotonic OCP of the single LiFePO4 particles.4,10,13 Therefore, particles in the two-phase region, referred here as the active population,14,15 sustain most of the applied current and have a strong influence on the rate performance. The first purpose of this study is to use the many-particle model along with the active population concept to explain the memory effect.
In addition to the memory effect, Farkhondeh et al.10 also simulated the path-dependence behavior and concluded that it could be explained by the same mechanisms as for the memory effect. This in contrast to the paper by Delacourt et al.9 based on the resistive reactant concept,16 which suggested that the path dependence originated from the inhomogeneous kinetics of particles caused by electronic resistance. In other words, the approach by Delacourt et al. suggests that the path dependence is independent of the non-monotonic potential profile. So, the second purpose of this manuscript is to revisit the path-dependence behavior and apply the many-particle model to describe this phenomenon and resolve the contradiction.
While the many-particle model has reproduced many of the unique phenomena in LiFePO4,10 in this paper we also identified a new phenomenon, that the voltage profile after relaxation is influenced by the relaxation time, with a larger polarization after a longer rest. We refer to this phenomenon as relaxation induced polarization (RIP). In this paper, we demonstrate this effect and explain its cause based on the many-particle model and the active population.
In summary, this study intends to elucidate the mechanisms and origins of multiple unique polarization behaviors of the LiFePO4 electrode using a unified model. We first clarified the polarizing mechanism underlying the memory effect. We then examined the RIP phenomenon and showed the ability of our model to explain this feature. We also investigated the main cause of the path dependence in LiFePO4 by simulating the dependence on the potential gap in the potential profile of a single LiFePO4 particle. Finally, we analyzed the effect of a narrower particle size distribution as a means of reducing these polarization behaviors.
Model
Based on the conventional single-particle model,17,18 we developed a many-particle model similar to that reported by Farkhondeh et al.10 The LiFePO4 electrode in this model consists of many distinct spherical particles connected electronically and ionically with each other (Fig. 1a). In previous reports, some of the polarization behaviors considered here were experimentally observed even in thin electrodes6,19 and at low current rates.5,19 Therefore, mass transport phenomena such as electronic conductivity and lithium diffusion in the solid and liquid phases are ignored in this model, and the Li concentration within the particle and in the electrode pores are considered to be uniform during the charge and discharge processes.
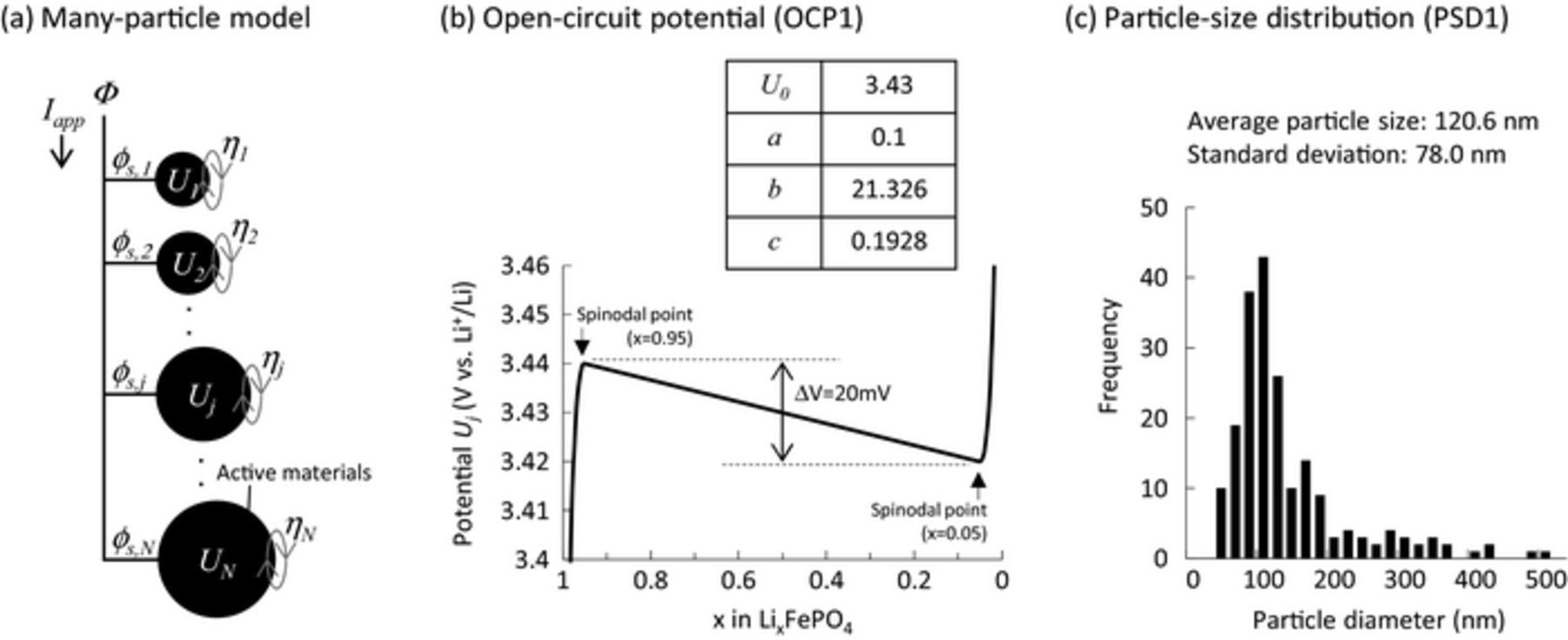
Figure 1. (a) Schematic representation of the many-particle model. (b) OCP profile used in this study. (c) Particle-size distribution with log-normal distribution for the particles comprising the LiFePO4 electrode.
The current in each particle is related to the change in Li concentration within it, due to the assumption of a uniform Li concentration in the particles. The Li concentration change in each particle is expressed by:
![Equation ([1])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0001.gif)
where j labels the individual particles (j = 1, 2, ..., N, where N is the total number of particles considered in this model), t is the time, F is the Faraday's constant, and ij is the current density for the jth particle. Sj is the surface area and Vj is the volume of the jth particle, both obtained from the particle radius (rj) as:
![Equation ([2])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0002.gif)
![Equation ([3])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0003.gif)
The total current in each particle should add to the applied current (Iapp):
![Equation ([4])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0004.gif)
The current density of lithium intercalation and deintercalation reactions for the jth particle is expressed by the Butler–Volmer equation:20
![Equation ([5])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0005.gif)
where k is the reaction rate constant, ce is the Li concentration in the electrolyte solution, cmax is the maximum Li concentration in the LiFePO4 particle, R is the gas constant, T represents the temperature, and ηj denotes the overpotential for the reaction. Here, we use 0.5 as the charge transfer coefficient for both the anodic and cathodic reactions.9,21 Although the charge-transfer coefficient can influence the reaction kinetics, the reaction order of many particles and any other factors related to the mechanisms discussed in this paper are not affected by it.
We think the electronic resistance of the LiFePO4 electrode is sufficiently low to be ignored, by assuming a carbon coating, a sufficiently large loading of conductive carbon, and a thin electrode. Polarization caused by the anode and separator is also assumed to be negligible. Therefore, the overpotential of the reaction for the jth particle is defined as:
![Equation ([6])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0006.gif)
where ϕs,j is the solid phase potential, ϕe,j is the liquid phase potential (here, ϕe,j = 0), and Uj is the OCP. The potentials of all particles correspond to the electrode potential (Φ):
![Equation ([7])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0007.gif)
In order to provide a more quantitative discussion, we designed the potential profile of single particles according to the results from galvanostatic intermittent titration technique (GITT) measurements (Fig. S1). The solid-solution regions of the LixFePO4 particles were found to be 1.0≥x≥0.95 and 0.05≥ x≥0.0. The OCP for a single particle of LiFePO4 was therefore constructed as:
![Equation ([8])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0008.gif)
where a, b, and c are constants, U0 is the equilibrium potential, and xj is the Li composition in LixjFePO4 for the jth particle and defined as:
![Equation ([9])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0009.gif)
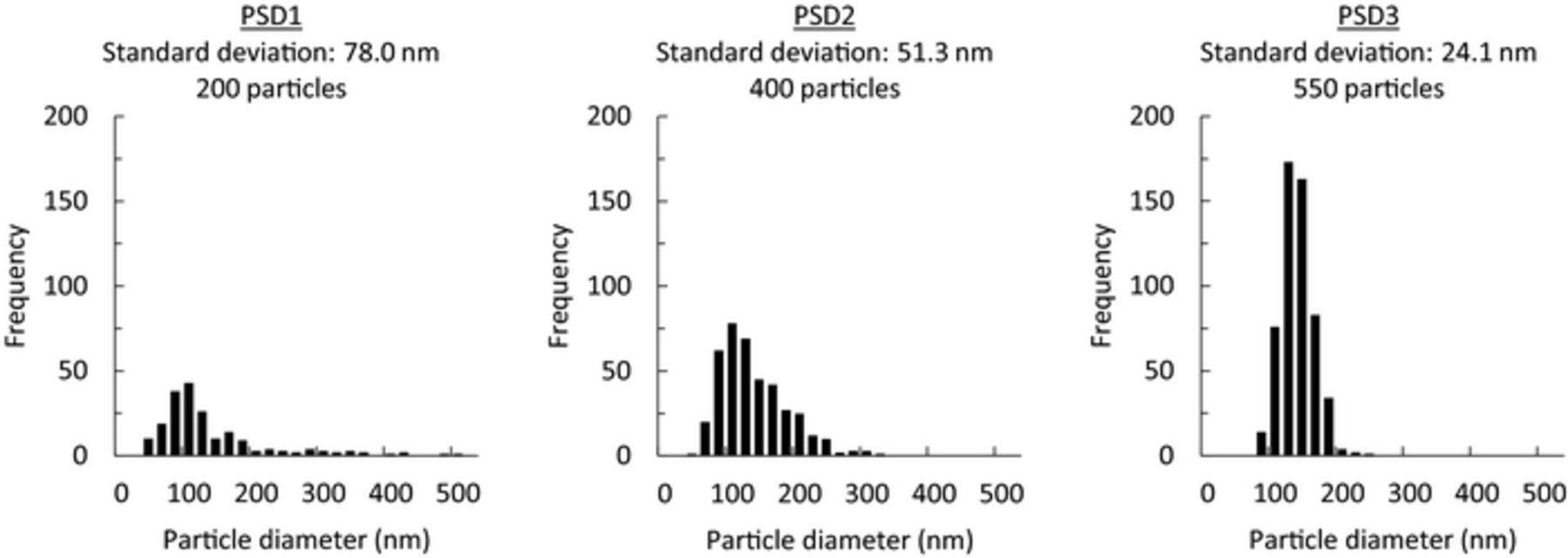
The OCP used in this study is shown in Fig. 1b with its parameters. The potential gap between two spinodal points is set to 20 mV, as reported from previous experiments.4 The average potential of the two spinodal points, which is the equilibrium potential if two phases coexist in the single particle,4 was estimated to 3.43 V vs. Li+/Li from GITT measurements (Fig. S1). In previous mathematical studies using the many-particle model, the electrochemical behavior of the LiFePO4 electrode was simulated in systems of 4 to 1000 particles.4,10,13 In this study, we first simulated the main polarization behaviors of the LiFePO4 electrode using the many-particle model with N = 200–5000. As shown in Fig. S2, all simulations showed the same qualitative results regardless of the number of particles. So, we decided to adopt 200 particles in the present many-particle model to save computational time. These particles have a lognormal size distribution, with an average size of 120 nm and standard deviation of 80 nm (see histogram in Fig. 1c). The dependence of particle size on the miscibility gap,22–24 potential gap,25 and equilibrium potential26,27 on the OCP curves are ignored in this model for simplicity. The solution procedure is described in Appendix A. Parameters used in this model are listed in Table I.
Results and Discussion
Memory effect
A simulation was carried out using non-monotonic potential profile with the potential gap of 20 mV as OCP for each single particle, and a total of 200 particles with average size of 120.8 nm and standard deviation of 78 nm (see Figs. 1b, 1c). These conditions were denoted as OCP1 and PSD1, respectively. Before investigating the memory effect, we simulated the voltage profiles and Li composition of each particle during normal charging and discharging to validate the model. The simulation results are shown in Fig. S3. Hysteresis behavior was observed even at a low rate (0.001C) of charging, as reported previously in studies using the many-particle model.10,13 In general, LiFePO4 electrodes consisting of many particles never react homogeneously owing to their distinct electronic connectivity and particle size. In this model, the electronic conductivity of the electrode was ignored, and only the particle size distribution was considered. Therefore, the reaction progress for the particles should differ depending on their size. Since smaller particles have a larger surface-area-to-volume ratio (Sj/Vj) than larger particles, their Li composition changes faster. Once the small particles overcome the potential barrier at the spinodal point and enter the two-phase region, they are preferentially delithiated to the Li-poor phase because of its lower potential (Fig. 1b). The phase transition for each particle during charging at low rate is shown in Fig. S3(b). Smaller serial numbers are given to particles with smaller sizes. During the charging process, it was confirmed that the smaller particles delithiated first from the Li-rich phase to the Li-poor phase and delithiation of the larger particles occurred later, as mentioned above. In practice, some particles with similar kinetic parameters react simultaneously, and the phase transition behavior shows a group-by-group sequence.13 On the other hand, when the LiFePO4 electrode was (dis)charged at a relatively high rate (Fig. S3(c) and (d)), most of the particles entered the two-phase region at the same time, and (dis)charging proceeded as a single-phase reaction (Fig. S3(d)). This means that the number of actively reacting particles in the two-phase region depends on the (dis)charging rate. Such rate dependence is consistent with previous experimental studies.15,28 The charge and discharge profiles at a relatively high rate showed arched shapes with potential overshooting at the beginning of charge and discharge. The overshooting originated from the non-monotonic potential profile and particle-by-particle charging behavior (see Fig. S3), and agreed with experimental observation.6
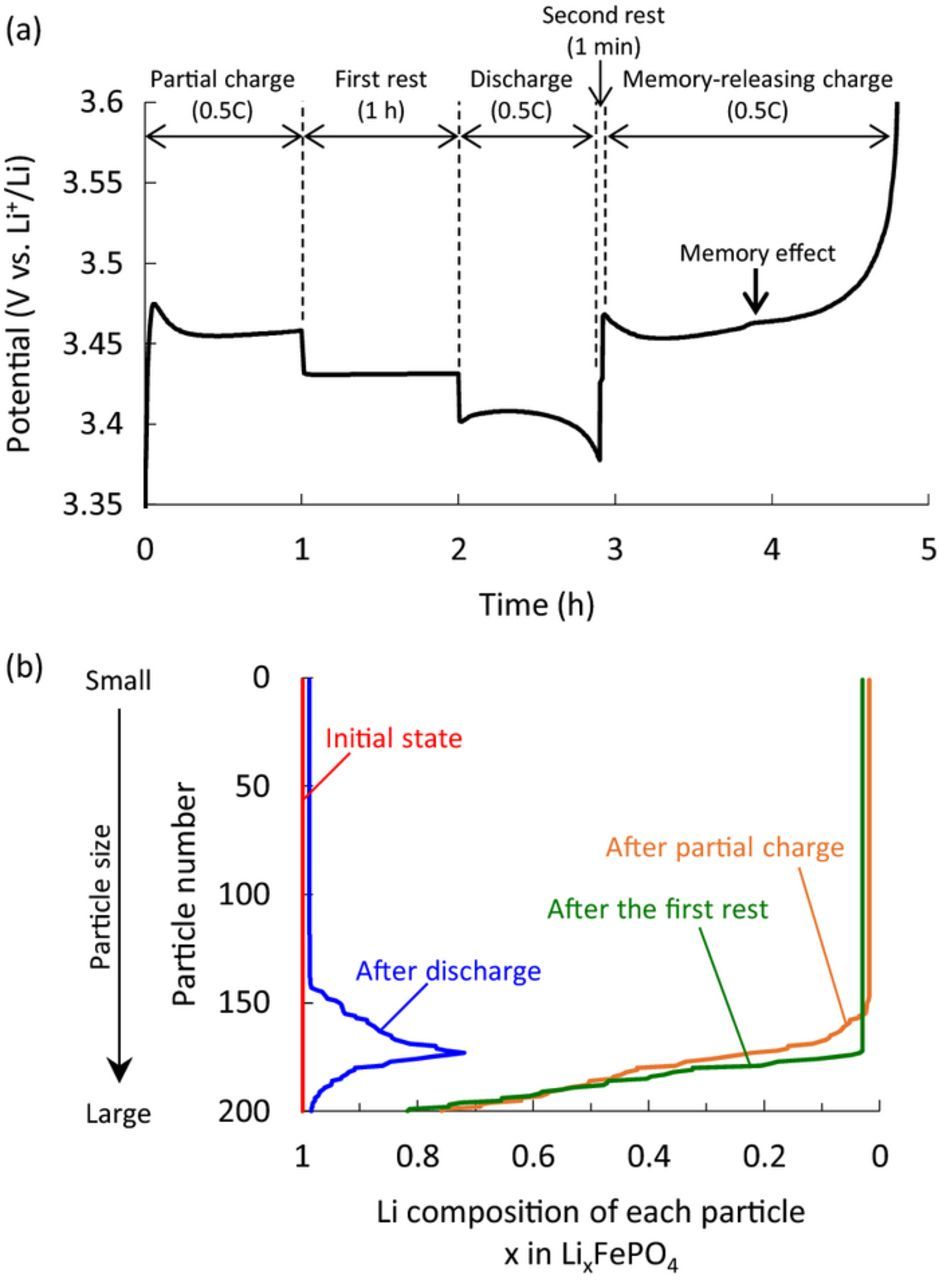
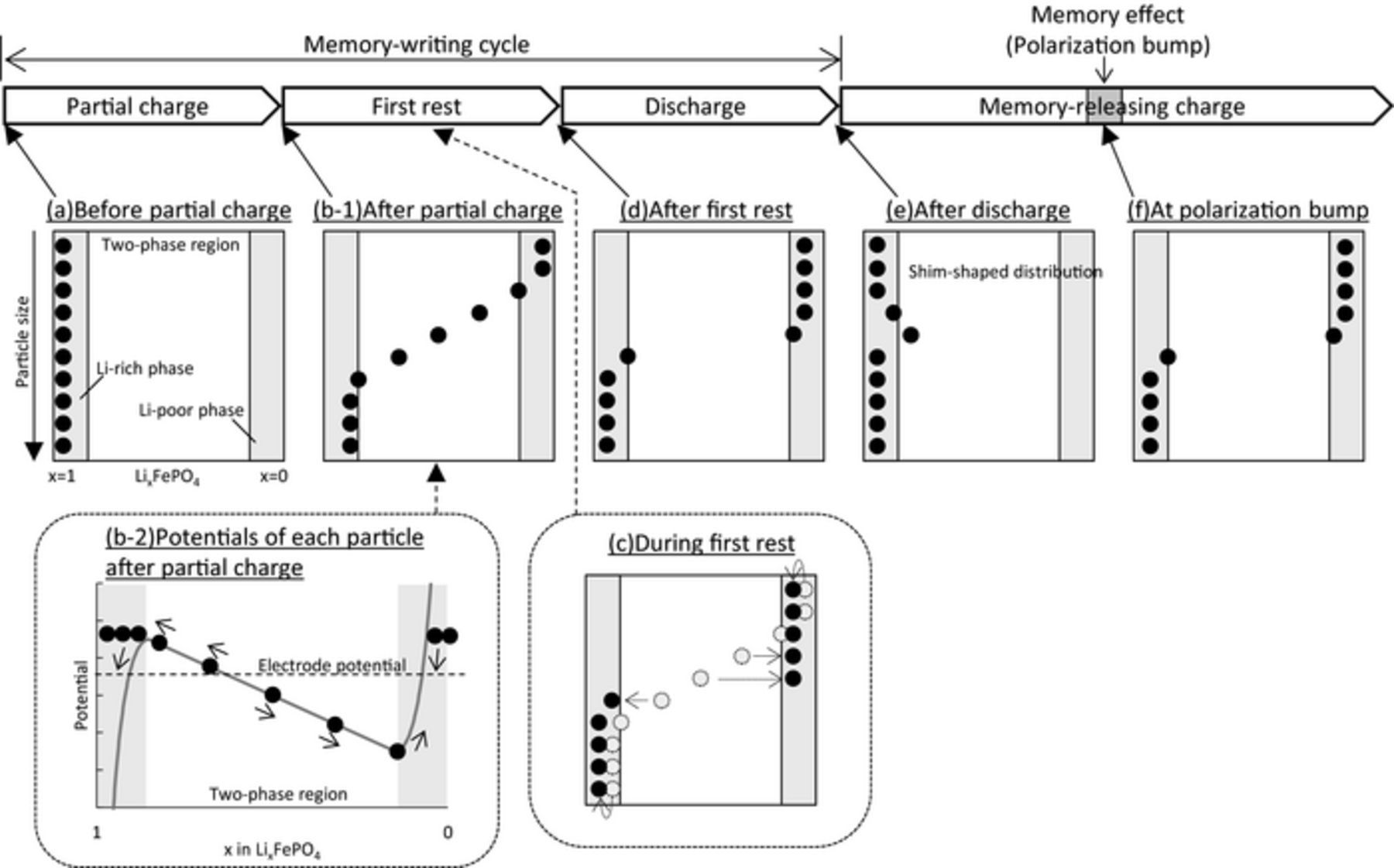
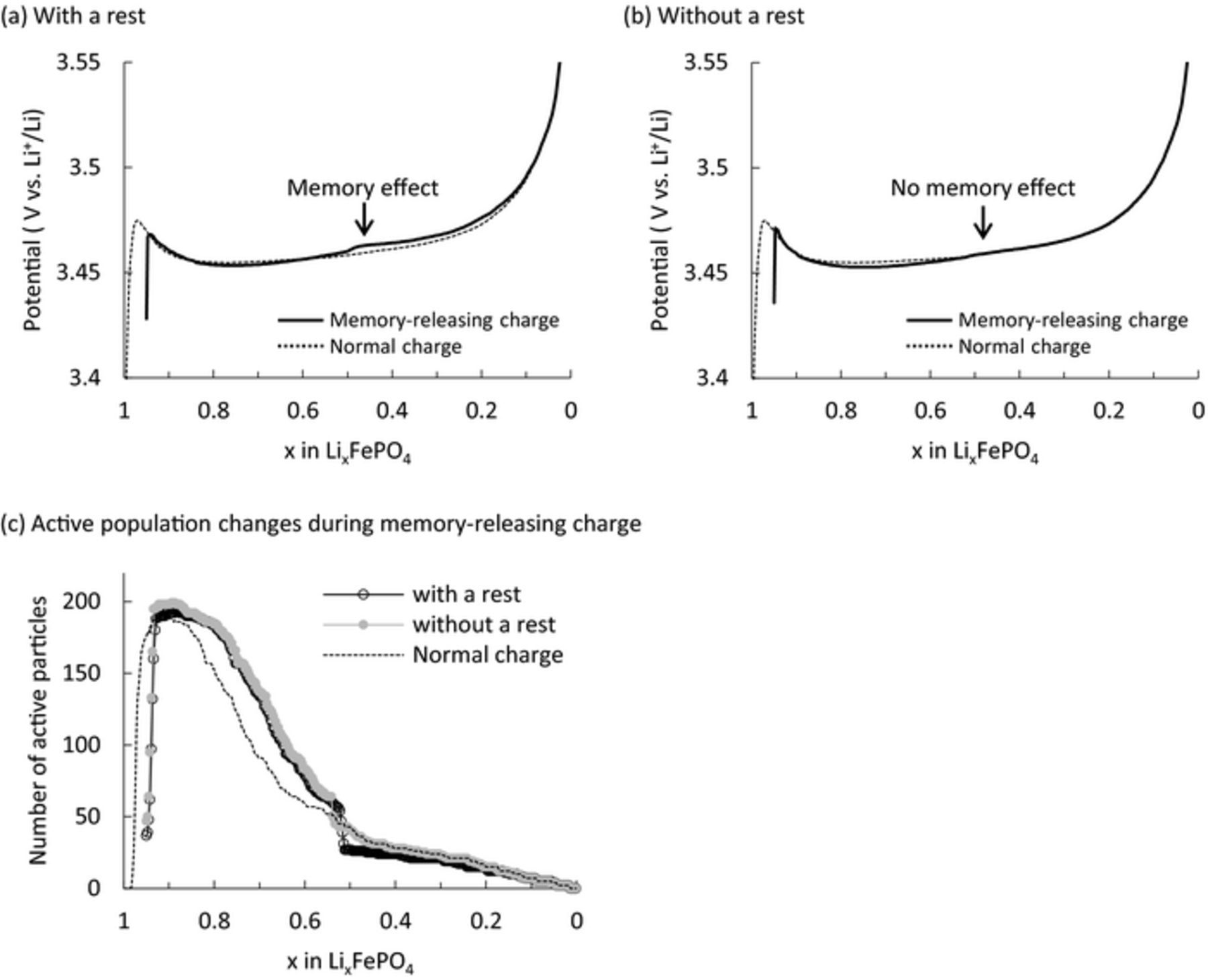
The memory effect was simulated using the following charge/discharge conditions according to Sasaki's report.6 First, the Li concentration of all particles was set to 22800 mol/m3 (x = 0.999 in LixFePO4) and the LiFePO4 electrode was partially charged to SOC50% at 0.5C. The electrode was then rested for 1 h (first rest) before discharge, and then rested again for 1 min (second rest). This sequence of steps comprises the memory-writing cycle. Finally, the electrode was charged (the memory-releasing charge). The simulation results are shown in Fig. 2a. An extra polarization bump of the memory effect was observed in the memory-releasing charge profile at nearly SOC50% where the initial partial charge was stopped. The Li composition of each particle at each step up to the discharge is shown in Fig. 2b. The Li-composition distribution for particles showed a shim shape after discharge (Fig. 2b, thick dotted line), as reported by Farkhondeh et al.10 To clarify the mechanism of this process, a schematic representation of the Li-composition change during the memory-effect processes is illustrated in Fig. 3. The charging started at the smaller particles as mentioned above. After the partial charge, the smaller particles reached the Li-poor phase and completed their phase transition, while the larger particles remained in the two-phase region or Li-rich phase (Fig. 3b-1). Here, the potentials of each particle on the OCV curve after the partial charge are shown in Fig. 3b-2. The electrode potential (indicated by horizontal dash line) is thought to be a mixed potential of all particles. The electrode then relaxed during the first rest, and the particles exchanged Li ions with each other to reach a single potential identical to that of the electrode. Since the LiFePO4 particles had the non-monotonic potential profile, which lead to the existence of two different concentrations with the same OCP, their Li compositions did not become identical but separated for each phase during the relaxation (Fig. 3b-2). Thus, the particles below the electrode potential were delithiated, and those above it were lithiated as indicated by arrows in Figs. 3b-2 and 3c. That is, among particles in the two-phase region, those below the electrode potential released the extra lithium. After the relaxation during the first rest, the particles lithiated again in the subsequent discharge starting with the smaller particles. Since some particles released extra lithium during the first rest, these particles formed a shim-shaped distribution after discharge (Fig. 3e). Farkhondeh et al. reported this unusual Li-composition distribution before the memory-releasing charge, and considered it to be the cause of the memory effect.10 However, it is not easy to intuitively understand how such a shim-shaped distribution leads to the polarization bump of the memory effect. To clarify this issue, we introduce the concept of "active population", as reported by Bai et al.14 In this concept, since the particles in the two-phase region have larger overpotential than those in the solid-solution region, most of the applied current is sustained by the actively reacting particles in the two-phase region. That is, the polarization is determined by the number of active particles (i.e., the active population).14,15 Using this concept, we deciphered the mechanisms for generating the extra polarization during the memory-releasing charge.
Figure 2. (a) Simulated potential profile of the memory effect and (b) Li composition change of each particle up to discharge.
Figure 3. Schematic representation of the memory effect mechanism describing the Li-composition change assuming 10 particles of different sizes. The gray areas correspond to the solid-solution region of the Li-rich and the Li-poor phase. (b-2) represents potentials of each particle after partial charge (before the first rest) on the OCP profile of LiFePO4.
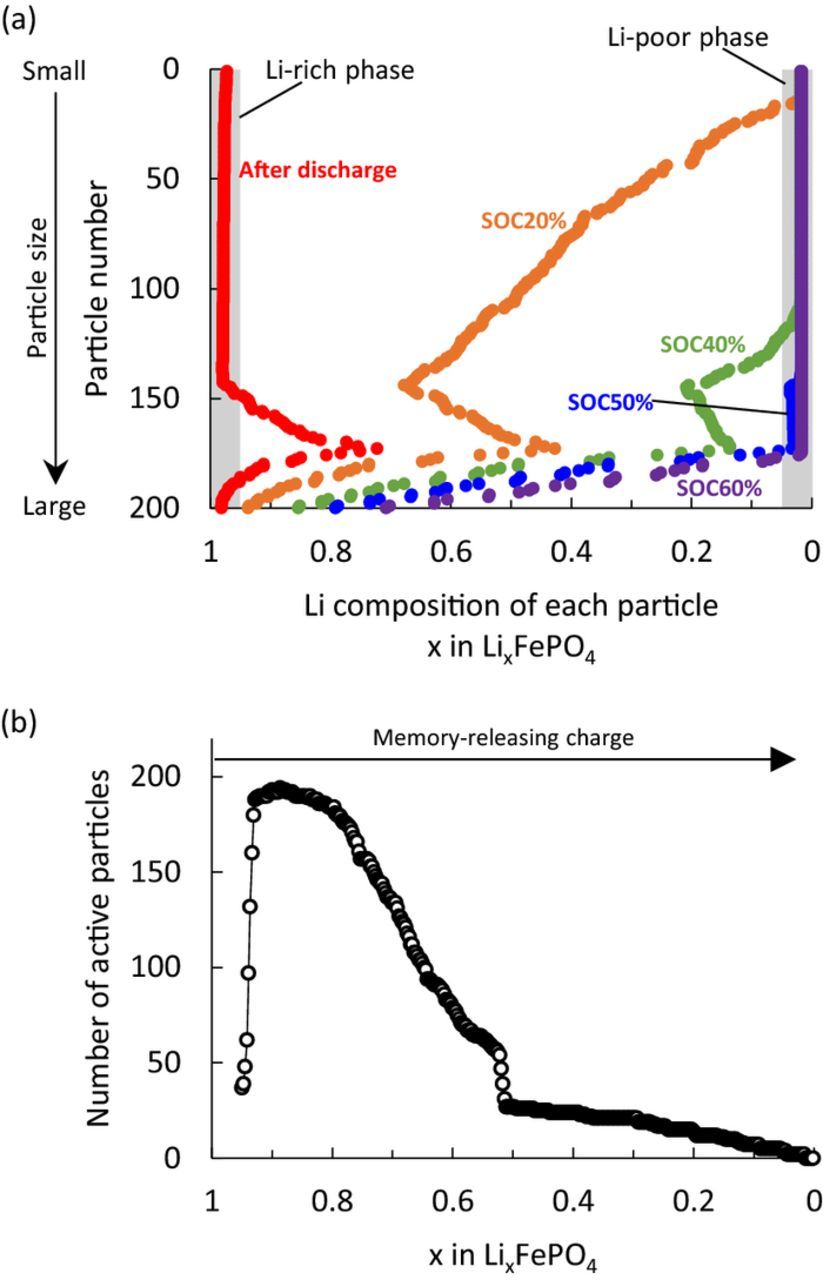
Changes in the Li composition for each particle during the memory-releasing charge are shown in Fig. 4a. At the beginning of this process (After discharge to ∼SOC20% in Fig. 4a), smaller particles were delithiated first. When the SOC changed from 40% to 50%, the particles corresponding to the one-sided shim wall completed their phase transition and entered the Li-poor phase region together, reducing the number of active particles in the two-phase region abruptly. This effect can also be directly seen in the active population change during the same period (Fig. 4b). The active population rose at the beginning of the memory-releasing charge, because particles entered the two-phase region from the Li-rich phase. Thereafter, the active population was reduced gradually in the early half of charging because the smaller particles reached the Li-poor phase region. The active population then suddenly dropped at SOC50% because a whole group of particles transformed to the inactive Li-poor phase (x<0.05) simultaneously, as seen in Fig. 4a. From these simulation results, we suggest that the sudden reduction of the active population is the cause of the polarization bump and is indicative of the memory effect.
Figure 4. (a) Li composition change at each SOC and (b) active population change in the memory-releasing charge.
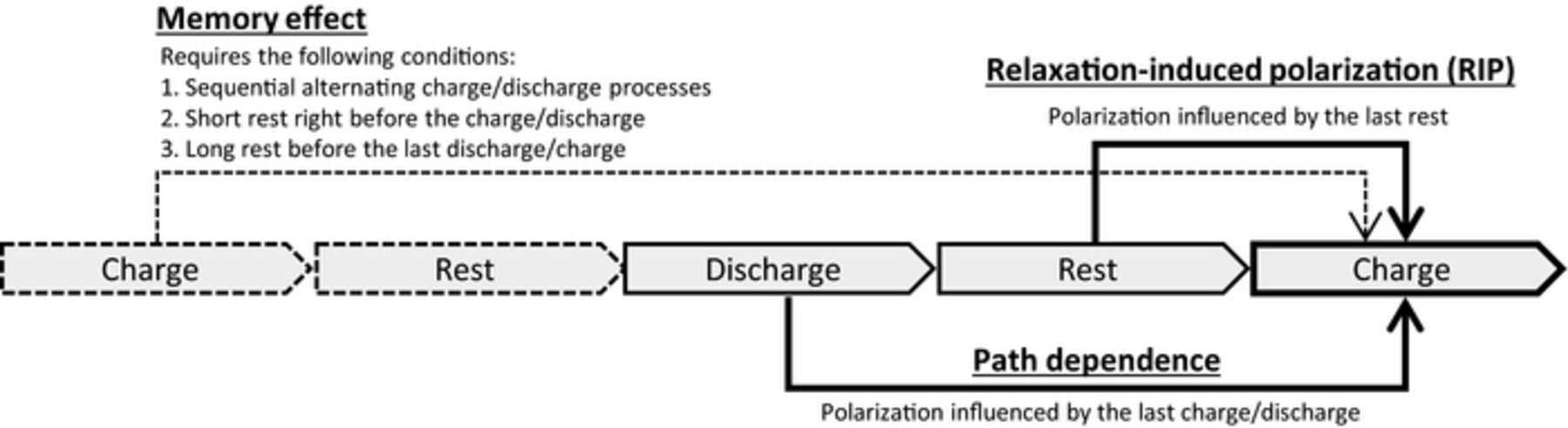
To clarify the intrinsic cause of the reduction in the active population during the memory-releasing cycle, the effect of each process on the memory effect was investigated. It was already reported that the second rest in the memory-writing cycle must be short to develop the memory effect, because the shim-shaped Li-composition distribution for particles can be relaxed and become flat.6,10 Therefore, we can ignore the second rest because it is too short, and summarize the Li-composition change of each particle in the scheme shown in Fig. 3. Discharging in the memory-writing cycle and charging in the memory-release cycle were performed sequentially without a rest (Fig. 3d ⇒ 3e ⇒ 3f). The Li-composition distribution after the first rest (Fig. 3d) should ideally be reproduced at SOC50% (Fig. 3f) in the memory-releasing charge. Here, the Li-composition distribution after the first rest was formed by relaxation during the first rest (Fig. 3b-2, 3c). In this relaxation process, some particles released Li ions and thus completed their phase transition to the Li-poor phase, whereas some others accepted Li ions and spontaneously returned to the Li-rich phase. When we examine this relaxation effect in Figs. 3b-2, 3c, we can say that the relaxation at the first rest reduced the active population of the LiFePO4 particles. That is, the active population at SOC50% on the memory-releasing charge was reduced by relaxation during the first rest, leading to increased polarization in the memory effect. Therefore, the primary cause of the memory effect is the reduction of the active population by relaxation during the first rest. As reported previously,6,10 the second rest is also important to maintain the reduction effect. However, the relaxation of the first rest is essential in the sense that it generates the memory effect by reducing the active population, which is directly linked to polarization. We identified three requirements for the memory effect on the basis of this mechanism. First, the memory effect needs a sequential four-step operation with alternating charge and discharge processes and a rest, namely charge-rest-discharge-(rest)-charge or discharge-rest-charge-(rest)-discharge. Second, the first rest must be long, and thirdly the second rest must be absent or short, as summarized in Fig. 12.
Figure 12. Schematic representation summarizing the unique polarization effects.
To confirm the necessity of the first rest, the memory effect with different rest times was simulated. The simulation results with and without a 1-h first rest are shown in Fig. 5. As expected, the polarization bump of the memory effect vanished when the first rest was absent (Fig. 5b). Figure 5c shows changes in the active population during the memory-releasing charge. The active population with and without the first rest changed similarly in the first half of the memory-releasing charge. At SOC50%, the active population with 1-h rest dropped below that with the normal charge, while that without a rest showed a small drop but never became lower than that with the normal charge. Therefore, polarization in the memory-releasing charge without the first rest never surpasses that with the normal charge (Fig. 5b). Recently, Jia et al.29 reported the dependence of the memory effect on the first rest time, and indicated that the relaxation strongly influenced the memory effect. Our simulation results agree with their experimental results.
Figure 5. Influence of the first rest time on the memory effect. Memory-releasing charging profiles (a) with and (b) without a 1-h rest for the first rest. (c) Active population change during the memory-releasing charge.
Relaxation-induced polarization
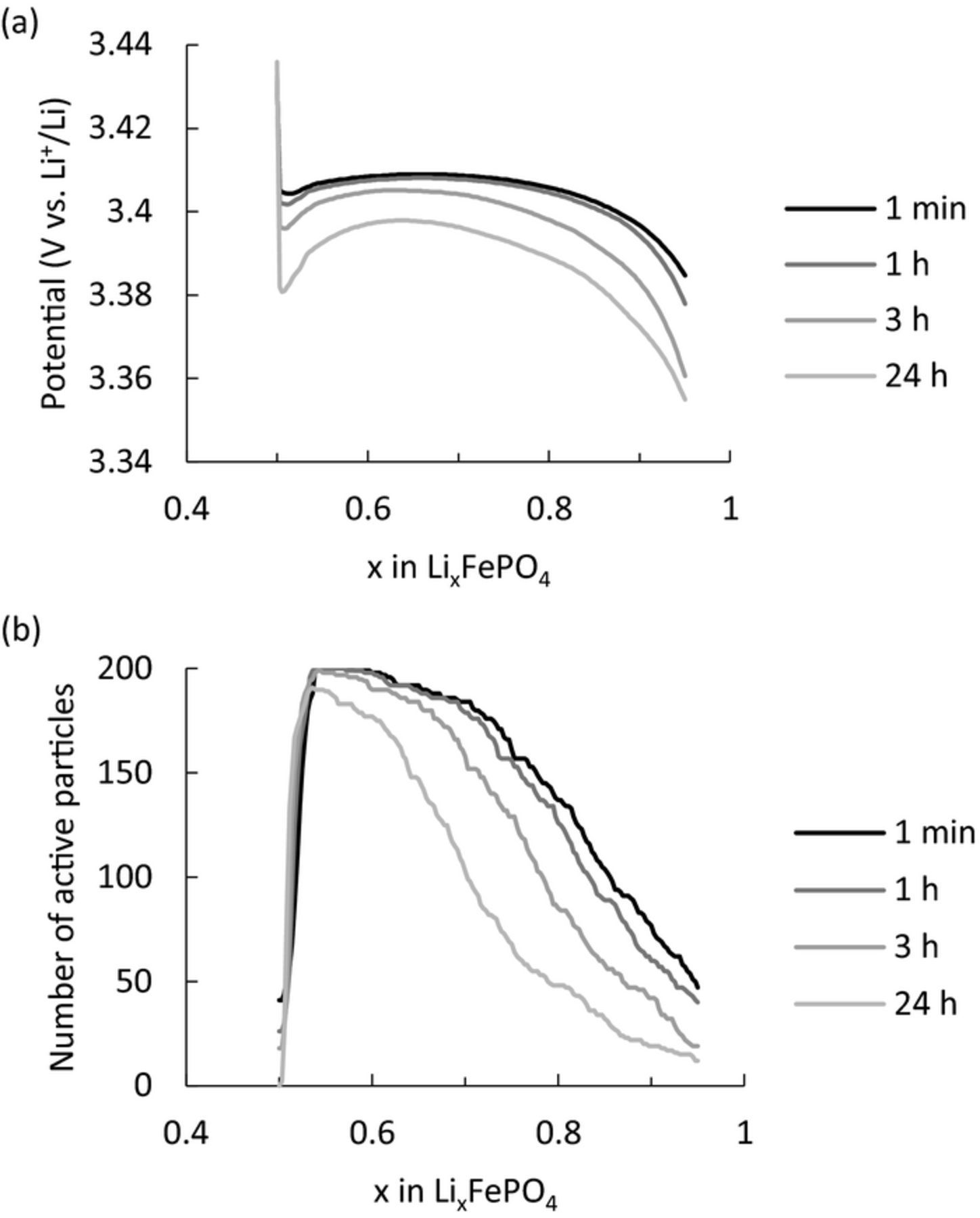
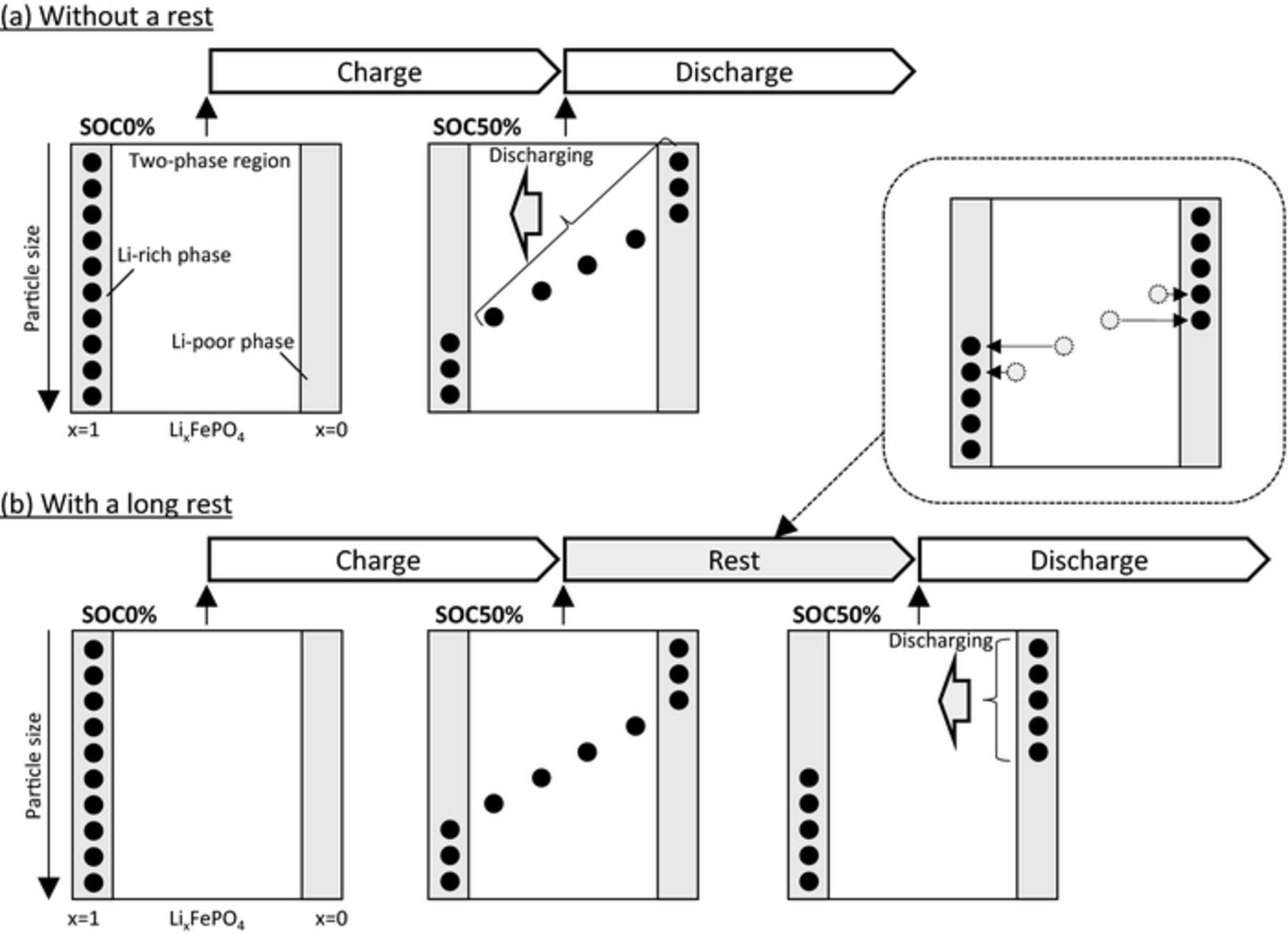
The proposed mechanism of the memory effect is that a reduction of the active population during the rest influences the polarization after a few steps. In terms of the active population, if relaxation in a rest reduces the active population, it should also affect the charge/discharge immediately after the rest. For example, in Fig. 4 the effect of reducing the active population during the first rest should affect the polarization of the next discharge. To this end, we investigated the polarization behavior of a LiFePO4 electrode after different rest times. The simulations used simple charge/discharge conditions identical to the memory-writing cycle as follows. The electrode was charged at 0.5C to SOC50% and then rested for different lengths of time (1 min, and 1, 3, and 24 h). After resting, the electrode was discharged at 0.5C. Discharge profiles and active population changes during the discharge are shown in Figs. 6a and 6b, respectively. As expected, the polarization increased with increasing rest time (Fig. 6a). The active population during discharge after a longer rest time was smaller (Fig. 6b). Proposed mechanisms of the polarization differences after different rest times are summarized schematically in Fig. 7. When the rest was absent or short (Fig. 7a), particles were distributed in three regions, namely the Li-rich phase, Li-poor phase, and two-phase regions after charging from SOC0% to SOC50%. In this case, particles in the Li-poor phase and two-phase regions can be discharged in the subsequent discharge process. In addition, the kinetically unfavorable large particles were already in the two-phase region. During a long rest (Fig. 7b), the Li-composition distribution became relaxed. Particles in the two-phase region exchange Li ions with each other and enter into each phase. Consequently, the relaxation reduced not only the active population but also the number of particles that can react in the subsequent discharge, as depicted in Fig. 7b. Therefore, a long rest increases the polarization in the discharge immediately after a rest. An experimental example of this polarization behavior as a function of rest is given in Jia's paper.29 They investigated the polarization during charging directly after a rest, and assumed the formation of a new phase to explain the effect of the rest. However, there is no evidence of a new phase. We conducted simulations under the same conditions as in their experiment (Fig. S4), and reproduced their results without forming any new phase. Ref. 29 might be the first experimental report of the RIP. However, their material showed only 120 mAh/g in capacity, whereas the theoretical capacity of LiFePO4 was 170 mAh/g. If this discrepancy is due to 30% impurities, this could possibly disturb the ideal relaxation process. Therefore, it is necessary to verify the RIP behavior using LiFePO4 material with practical quality.
Figure 6. Influence of the rest time directly before the discharge. (a) Discharge profiles and (b) active population after different rest times.
Figure 7. Schematic representation of the mechanism of relaxation-induced polarization (RIP). (a) In the no-rest condition, seven particles can react in the discharge. Moreover, kinetically unfavorable large particles are in the two-phase region at the start of the discharge. On the other hand, for (b) with the long rest condition, only five particles can react in the discharge, and all particles exist in the solid-solution region.
The effects of relaxation on the polarization and memory effect have the same cause, namely a reduction of the active population. However, the trigger conditions were quite different. While the relaxation influences the memory effect under a particular set of conditions, as mentioned in Section Memory effect, the relaxation effect described in this section does not require any special conditions: it simply influences the charge/discharge that directly follows the rest. We call this relaxation effect "relaxation-induced polarization" (RIP). The mechanisms of both RIP and the memory effect suggest that the latter phenomenon is a particular case of the former. Large polarization overshooting observed in the GITT (dis)charging profiles reported by Sasaki et al.6 was thought to be a consequence of RIP. That is, the disappearance of active particles in the two-phase region during each rest is the reason for the overshooting.
Researchers had believed that the LiFePO4 electrode was not relaxed when the Li composition was in the two-phase region, because the very flat potential profile could not supply any driving force for relaxation. However, the potential of a single particle in the two-phase region is not flat but sloped,4,11,12 like OCP1 in Fig. 1b. Hence, relaxation could indeed occur in this region. Moreover, since the potential slope in the two-phase region is opposite to that in the solid-solution region against Li composition, the Li compositions of the particles never become identical during relaxation, and the particles separate into each phase (Fig. 3b-2). Therefore, it can be understood that the non-monotonic OCP caused both the memory effect and RIP.
Path dependence and influence of the potential gap in OCP
Next, we focus on the path-dependence behavior in addition to the memory effect and RIP as another essential polarization of the LiFePO4 electrode, and clarify the mechanistic differences among these phenomena. As mentioned in the Introduction, path-dependence behavior is characterized by a change in polarization due to the previous charge or discharge even if the initial SOC is identical.5
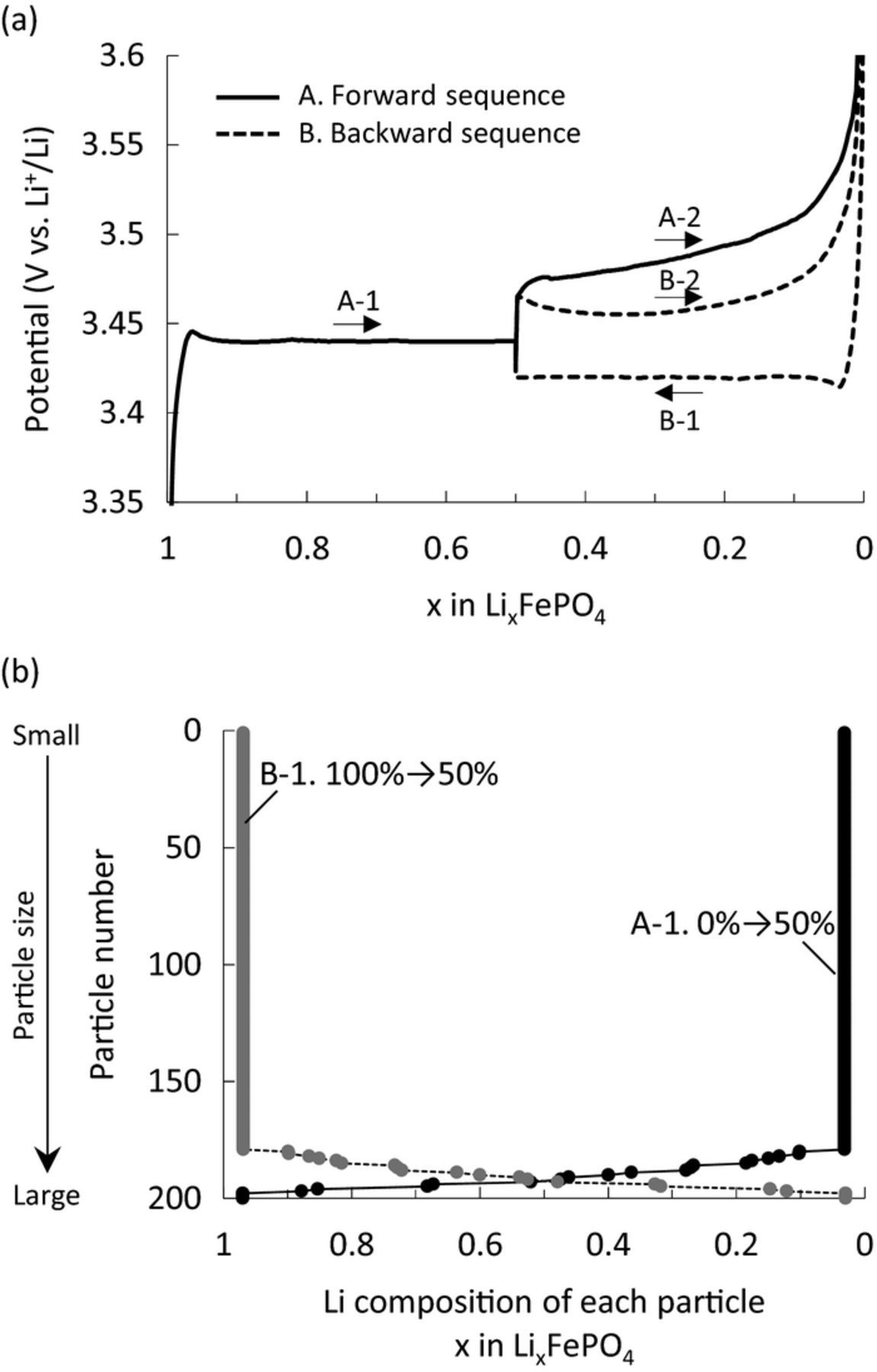
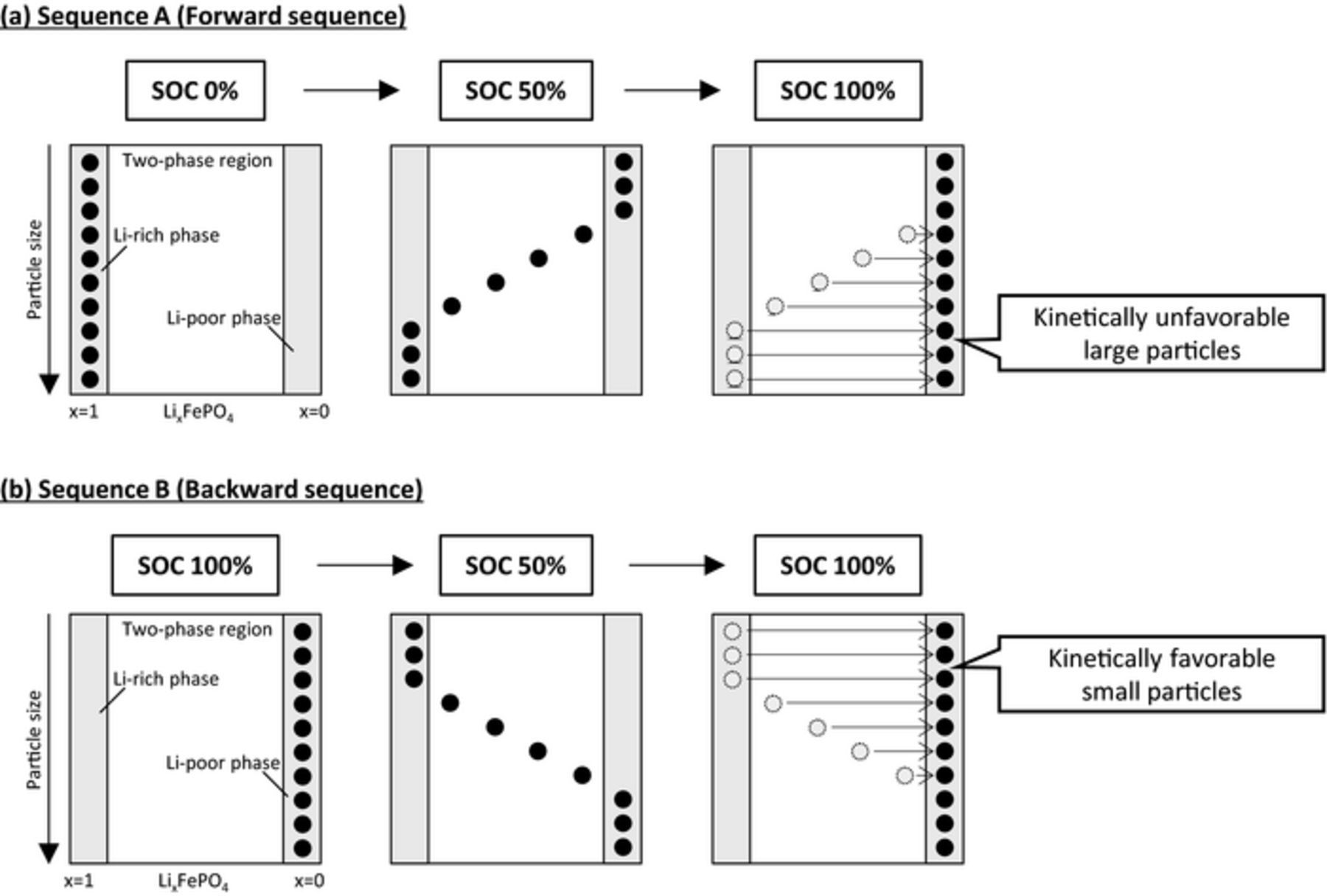
Two simulations with different conditions were compared to investigate the path dependence. For the charge/discharge sequence A (forward sequence), the electrode was charged from SOC0% to SOC50% at 0.1C rate, and after 1-h rest the electrode was charged to SOC100% at 0.5C. For the other charge/discharge sequence B (backward sequence), the electrode was discharged from SOC100% to SOC50% at 0.1C rate, and after a 1-h rest the electrode was charged to SOC100% at 0.5C. The final charging processes from SOC50% to SOC100% under these two conditions were compared, and typical simulation results are shown in Fig. 8a. Charging from SOC50% to SOC100% after charging (forward sequence A) showed larger polarization than that after discharging (backward sequence B), as reported in the literature,5,19 even though the compared processes were identical (charging from SOC50% to SOC100%). The Li-composition distributions for both sequences at SOC50% are symmetrical, as shown in Fig. 8b. From the simulation results, we verified the mechanism of the path-dependence behavior of the LiFePO4 electrode, as described in Fig. 9. Since the particles reacted according to their size in this model, the smaller particles reacted first regardless of the starting SOC. In the forward sequence A (Fig. 9a), kinetically favorable small particles had completed phase transition to the Li-poor phase at SOC50%, therefore kinetically unfavorable large particles, whose phase transition remained incomplete, must react in the following charge. In the backward sequence B (Fig. 9b), kinetically favorable small particles reached the Li-rich phase at SOC50% and remained available to react in the final charge. Therefore, sequence A, which left kinetically unfavorable particles for charging, showed larger polarization than sequence B, in which kinetically favorable small particles can be charged. In summary, the mechanism of the path dependence was considered to involve inhomogeneous reaction kinetics among the particles, which is different from the mechanism of the memory effect and RIP.
Figure 8. Simulation results of typical path dependence conditions. (a) Charge and discharge profiles of the forward sequence (A. 0%–50%–100%) and backward sequence (B. 100%–50%–100%). (b) Li composition of particles at SOC50% after charging from a fully discharged state (A-1, 0%–50%) and discharging from a fully charged state (B-1, 100%–50%).
Figure 9. Schematic representation of the mechanistic differences in the polarization of the charge/discharge profiles according to the charge/discharge history (path dependence). (a) In the forward sequence, kinetically unfavorable large particles have to react in the charging from SOC50% to SOC100%. On the other hand, for (b) with the backward sequence, kinetically favorable small particles can react in the charging from SOC50% to SOC100%.
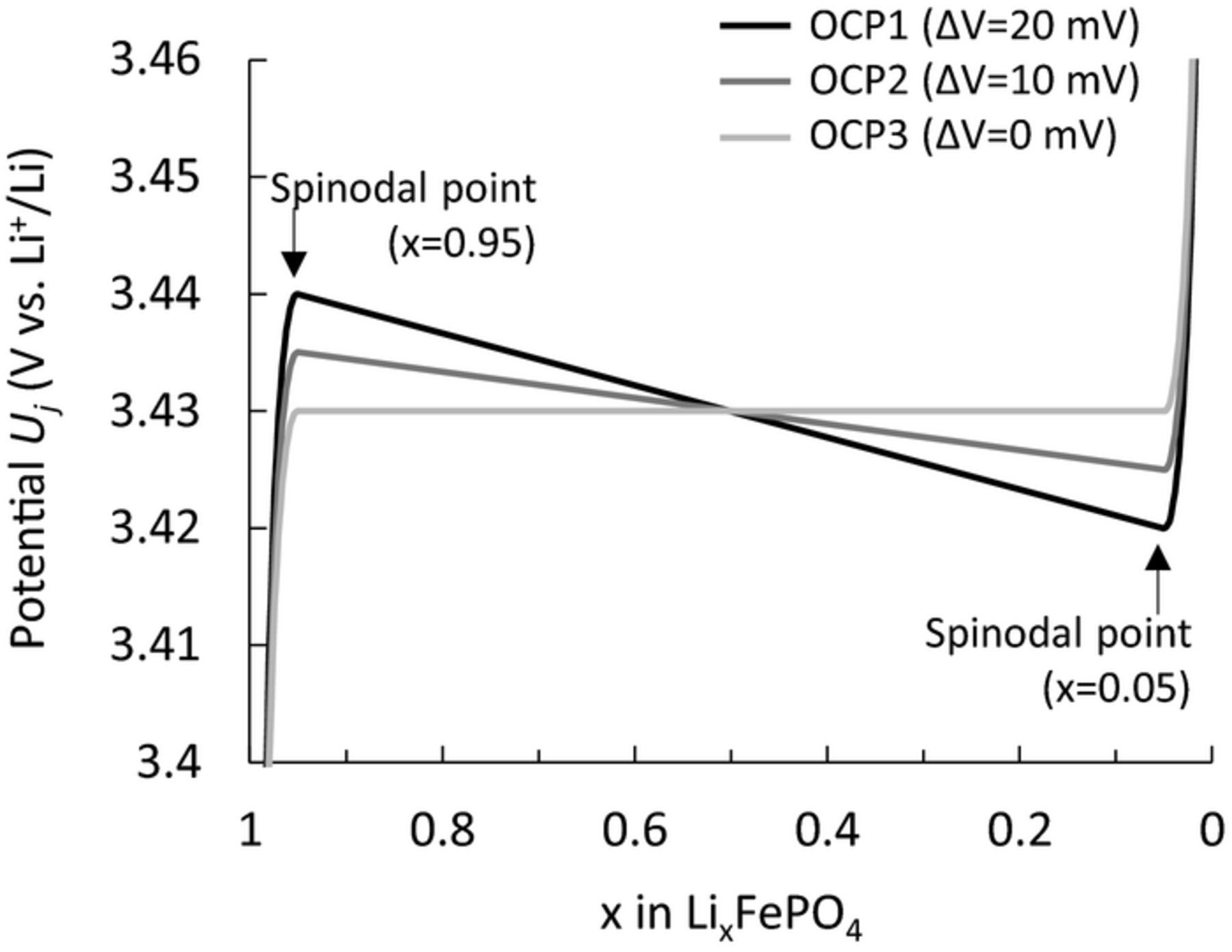
We also carried out a simulation to determine the influences of the shape of the OCP of a LiFePO4 single particle on the unique polarization behavior, including the path dependence to further support the proposed mechanisms. The non-monotonic (i.e., with slope) potential profile of the particle in the two-phase region is essential for the memory effect and RIP, as mentioned in Section Relaxation-induced polarization. It is known that the parameters that characterize the OCP profiles (i.e., the miscibility gap, potential gap between spinodal points, and equilibrium potential) depend on the particle size and shape.22–27 We have ignored the particle size dependencies of the OCPs in this model, because the quantitative relationship between the OCP and the LiFePO4 particle size was unclear. In this section, therefore, we generated OCPs with different potential gaps and simulated the memory effect, RIP, and path-dependence behavior. Three OCPs for a LiFePO4 single particle (OCP1–OCP3, Fig. 10) were prepared for the simulations, with the same miscibility gap and equilibrium potential but different potential gaps between two spinodal points located at the boundaries of the solid-solution and the two-phase regions. The parameters of these potential profiles are shown in Table II. OCP1 in Fig. 10 corresponds to that in Fig. 1b discussed earlier. The other two potential profiles had potential gaps of 10 mV (OCP2) and 0 mV (OCP3).
Figure 10. OCP profiles used to simulate the dependence of the potential gap in OCPs on the unique polarization.
Table II. Parameters of OCPs.
| Symbol | OCP1 | OCP2 | OCP3 |
|---|---|---|---|
| Potential gap | 20 mVa | 10 mV | 0 mV |
| U0 [V vs. Li+/Li] | 3.43b | 3.43 | 3.43 |
| a | 0.1b | 0.1 | 0.1 |
| b | 21.326b | 21.326 | 21.326 |
| c | 0.1928b | 0.1904 | 0.1881 |
aRef. 7. bEstimated from GITT data.
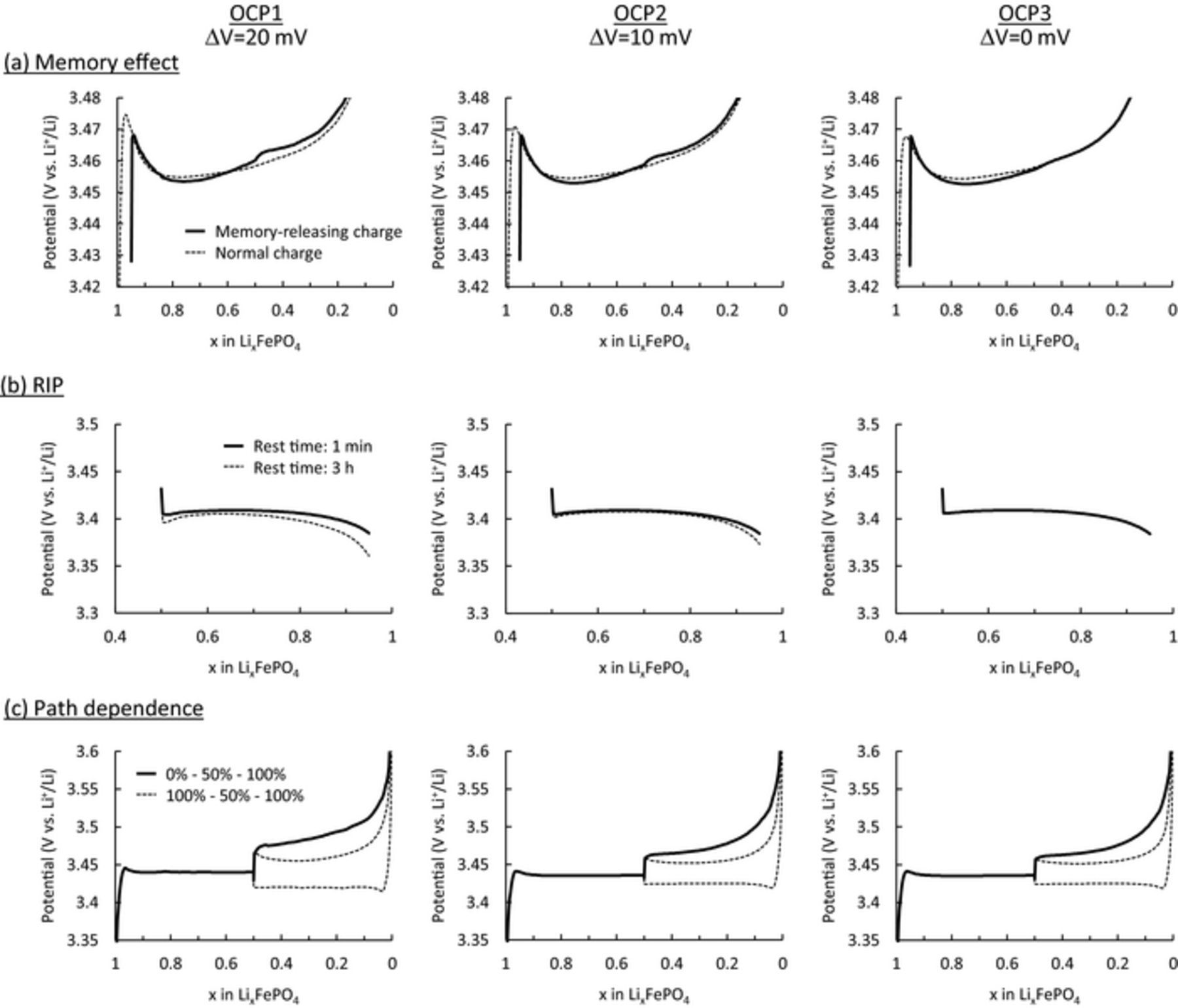
Simulations of the memory effect, RIP, and path dependence were carried out using these three OCPs, and the results are summarized in Fig. 11. The polarizations of the memory effect and RIP were reduced as the potential gap became smaller, disappearing at zero gap (OCP3). These results demonstrate that the essential factor of these two behaviors is the non-monotonic potential in OCP. In contrast, path-dependence behavior was not significantly influenced by changes in the OCP, even for the case with a zero-potential gap, although the two charge profiles became more similar in their polarization. The cause of the path dependence of the LiFePO4 electrode was kinetically inhomogeneous reactions. Because differences in the kinetic properties of particles are independent of the OCP, polarization of the path dependence was not influenced. These results proved that the cause of the path dependence differs from that of both the memory effect and RIP.
Figure 11. Influence of the potential gap in OCPs on the charge/discharge curves of the (a) memory effect, (b) relaxation-induced polarization (RIP), and (c) path dependence.
Based on our mathematical simulations using the many-particle model, a mechanism of the unique polarization behaviors of the LiFePO4 electrode is proposed (Fig. 12). The RIP and path dependence are particularly important to the charge/discharge behavior of the LiFePO4 electrode, because they always influence the charge/discharge polarization.
Effect of particle-size distribution
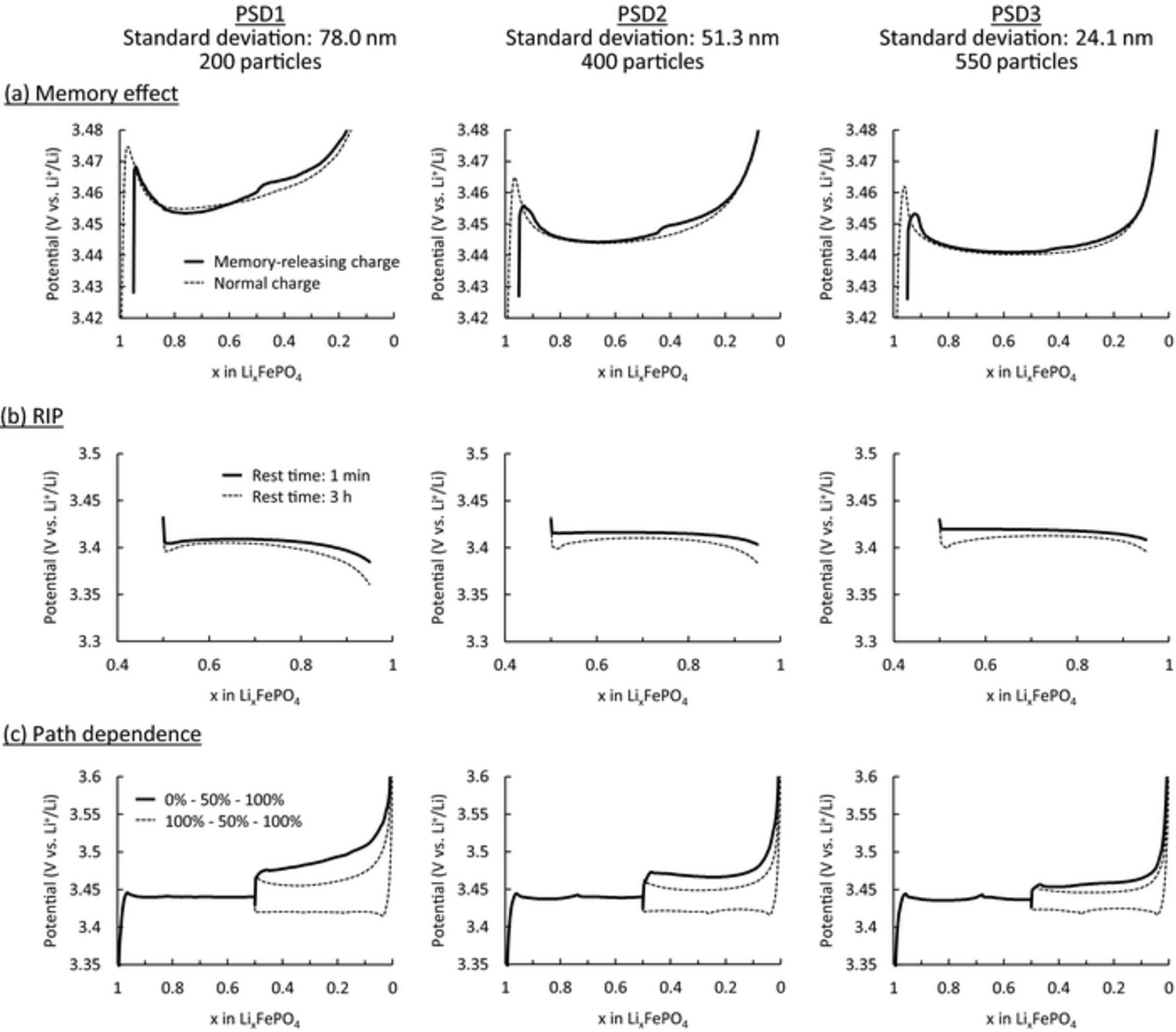
Although the unusual polarization investigated in this study was not very large (∼30 mV), polarization cannot be ignored when estimating SOC and state-of-health (SOH) because of the almost flat voltage profile of the LiFePO4 electrode.30,31 Therefore, it is important to minimize the polarization effect in practical applications. In this section, we investigated the influence of the particle size distribution on the polarization of LiFePO4 electrode. The simulations in the former sections were carried out for 200 particles using PSD1 (Fig. 1c), which had an average particle diameter of 120.6 nm and standard deviation of 78.0 nm. We prepared two other particle size distributions with identical average particle size and total particle volume but different standard deviations in size and particle numbers (PSD2: 51.3 nm and 400 particles, PSD3: 24.1 nm and 550 particles). Histograms of the particle size distribution are shown in Fig. 13, and the parameters are listed in Table III. It is noted that the total surface area, which strongly influences the reaction kinetics, differs for PSD1–PSD3 (Table III). OCP1 with a potential gap of 20 mV was used as the OCP profile. The simulation results of the memory effect, RIP, and path dependence are summarized in Fig. 14. Polarization of the memory effect was reduced by narrowing the particle size distribution (Fig. 14a). Considering the mechanism of the memory effect, these results are strange because the particle size is independent of the OCP profile. The active population change, shown in Fig. S5, clearly shows that the active populations of PSD2 and PSD3 also dropped at nearly SOC50% as in PSD1. So, this characteristic reduction of the active population, which directly causes the polarization bump in the memory effect, did not change even with particle size distribution. Therefore, amelioration of the memory effect with a narrower particle size distribution comes from the larger total surface area of the particles (Table III), which would reduce the overall polarization. This effect lowered the polarization of the memory-releasing charge, making the polarization bump difficult to recognize. However, despite this reduced polarization of the memory effect, the inherent "memory" was not eliminated.
Figure 13. Conditions used to simulate the dependence on the particle size distribution.
Table III. Parameters of particle-size distributions.
| Symbol | PSD1 | PSD2 | PSD3 |
|---|---|---|---|
| Average diameter [nm] | 120.6 | 122.2 | 123.0 |
| Standard deviation [nm] | 78.0 | 51.3 | 24.1 |
| Number of particles | 200 | 400 | 550 |
| Total surface area [nm2] | 1.40 × 107 | 2.19 × 107 | 2.71 × 107 |
| Total volume [nm3] | 5.98 × 108 | 5.98 × 108 | 5.98 × 108 |
Figure 14. Influence of the potential gap on the charge/discharge curves of the (a) memory effect, (b) relaxation-induced polarization, and (c) path dependence.
Regardless of the particle size distribution, the polarization differences of RIP between short and long rest times remained almost identical, whereas the dependence of the Li composition on these polarization differences changed (Fig. 14b). Since the essential cause of RIP is relaxation due to the non-monotonic potential profile of LiFePO4, the polarization differences in RIP did not change. In contrast, the path dependence was reduced drastically by narrowing the particle size distribution (Fig. 14c), and the polarization differences in charging between the forward and backward sequences were reduced. Since a narrower particle size distribution means more homogeneous reactivity of the particles, it reduced the kinetic inhomogeneity of the particles and led to an attenuation of the path-dependence effect. From the simulation results, it was found that a narrow particle size distribution was effective in reducing the polarization associated with the memory effect and path dependence; however, it had little influence on the RIP.
In the above discussion, only the particle size distribution in the model was varied to introduce local inhomogeneity in reaction. This assumption was thought to be appropriate because the unique polarization behaviors of LiFePO4 electrodes, such as the memory effect and path dependence, were experimentally observed even in thin electrodes6,19 and at low current rates.5,6 In thick or less conductive electrodes, mass transport in the solid and liquid phases bring inhomogeneity in addition to that from the particle size distribution. Consequently, the polarization behavior becomes complicated, and the unique polarization phenomena are likely be difficult to characterize, e.g. being obscured in polarization changes caused by the additional Li-concentration distribution in the solid and/or liquid phase from inhomogeneous reactions on the electrode scale.
Conclusions
Due to its particular two-phase reaction mechanisms, LiFePO4 is known to exhibit two unique polarization behaviors, namely the memory effect and path dependence. In this study, mathematical simulations were performed using a many-particle model to clarify the cause of these unique polarization behaviors. We also proposed a new polarization behavior termed "relaxation-induced polarization" (RIP), which has a similar origin as the memory effect. The influences of the OCP profile and particle size distribution on the polarization behaviors were also investigated.
Memory effect in the LiFePO4 electrodes was successfully explained in terms of the many-particle model and the active population concept. LiFePO4 particles can be relaxed and exchange Li ions among themselves due to their non-uniform OCP. Consequently, the relaxation occurring during the rest reduces the active population and causes an unusual polarization bump at a particular SOC in the memory-releasing (dis)charge. Likewise, RIP, which is caused by a reduction in the active population during relaxation, was predicted as a new type of polarization behavior. In contrast to the memory effect that is triggered by relaxation and appears in four steps, RIP affects the charge and discharge processes immediately after rest. In addition, whereas the memory effect requires particular conditions to establish and maintain the relaxation effect to induce the polarization bump, RIP depends only on the rest time. Therefore, RIP is essential for understanding the practical polarization of LiFePO4 electrodes.
Path dependence, in which the previous charge/discharge influences the polarization of subsequent charge/discharges, is another unique polarization behavior of LiFePO4 electrodes, and it always affects the polarization of the following charge/discharge. In the many-particle model, the cause of path dependence was the inhomogeneous reactivity of particles due to their different kinetic environments, i.e. particle size in this study. The dependence of the OCP profiles gave further evidence for the proposed mechanisms.
In order to reduce the unique polarizations of the LiFePO4 electrode, the effect of narrowing the particle size distribution was investigated. The simulations predicted that this would diminish polarization from the memory effect and path dependence. However, polarization from the RIP was only slightly reduced. A comprehensive understanding of polarization behaviors using our model and the active population concept will allow more accurate estimation of the state of charge of Li-ion batteries with LiFePO4 electrodes.
List of Symbols
| a,b,c | Constants for open-circuit potential |
| cj | Li+ concentration in the jth particle [mol/m3] |
| ce | Li+ concentration of electrolyte solution [mol/m3] |
| cmax | Maximum Li concentration in the active material [mol/m3] |
| F | Faraday's constant (96485 [C/mol]) |
| ij | Current density on the surface of the jth particle [A/m2] |
| Iapp | Applied current [A] |
| k | Rate constant of the reaction [m2.5/mol0.5 s] |
| N | Number of particles [-] |
| rj | Particle radius of the jth particle [m] |
| R | Gas constant (8.314 [J/mol·K]) |
| Sj | Surface area of the jth particle [m2] |
| t | Time [s] |
| T | Temperature [K] |
| Uj | Open-circuit potential of the jth particle [V (vs. Li+/Li)] |
| U0 | Equilibrium potential in the two-phase coexistence [V (vs. Li+/Li)] |
| Vj | Volume of the jth particle [m3] |
| xj | Li composition of the jth particle [-] |
Greek
| ϕs,j | Potential of solid phase for the jth particle [V (vs. Li+/Li)] |
| ϕe,j | Potential of liquid phase around the jth particle [V (vs. Li+/Li)] |
| Φ | Electrode potential [V (vs. Li+/Li)] |
| ηj | Overpotential for the jth particle [V] |
Subscripts
| j | Particle number (j = 1,2,...,N) |
| s | Solid phase |
| e | Liquid phase |
ORCID
Hiroki Kondo 0000-0002-2708-9039
Venkat Srinivasan 0000-0002-1248-5952
: Appendix A
This appendix details the method to solve the many-particle model presented herein. In this model, the total current for each particle must correspond to the applied current (Iapp) for the LiFePO4 electrode. The current density of each particle (ij) is obtained by Equation 5, using the overpotential for each particle (ηj) that was defined separately by Equation 6 using the particle potential (ϕs,j) and OCP (Uj) for each particle. Here, all ϕs,j values must be identical to the electrode potential (Φ), because the electronic resistance in the solid phase is ignored in this model. Therefore, the applied current is expressed as:
![Equation ([A1])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0010.gif)
Equation A1 is written using Equations 5, 6, and 7 as:
![Equation ([A2])](https://content.cld.iop.org/journals/1945-7111/165/10/A2047/revision1/d0011.gif)
Equation A2 is a nonlinear equation that contains only one unknown (Φ) and is solved using the Newton-Raphson method. The overpotential for each particle was obtained by Equations 6 and 7, and then the Li concentration in the particle was calculated by Equations 5 and 1.