Abstract
Ultrananocrystalline diamond (UNCD)/nonhydrogenated nitrogenated amorphous carbon (a-C:N) composite (UNCD/a-C:N) films were prepared on titanium substrates in a nitrogen atmosphere by pulsed laser deposition, and their adhesion to the titanium substrates was remarkably improved as compared with those of UNCD/hydrogenated nitrogenized amorphous carbon (a-C:H:N) composite films deposited in a nitrogen and hydrogen mixture atmosphere. Their chemical bonding structures were investigated in details by X-ray photoemission and near edge X-ray absorption fine structure spectroscopies with synchrotron radiation. The UNCD/a-C:N films exhibit an obviously enhanced formation of sp2 and sp bonding such as C=N and C≡N bonds. It should be contributable to the adhesion improvement. Since its formation locally disconnects sp3 bonding networks in the films, an internal stress, which is the main reason for exfoliation, should be released.
Export citation and abstract BibTeX RIS
Hard carbon is a promising coating material. Diamond possesses a high potential due to a variety of excellent physical properties, however the direct deposition of diamond films on metal substrates is limited due to their weak adhesion to the substrates and a large internal stress caused by a large mismatch in the thermal expansion coefficient between the films and substrates. In order to overcome their problems, interlayers between diamond films and metal substrates that strengthen adhesion at the interfaces and release an internal stress accumulated in diamond films, have been adapted by several groups.1–7 Diamond-like carbon (DLC) that possesses similar physical properties is also a candidate for coating. Lie et al. reported that DLC films have a higher internal stress than diamond films. Although the nitrogenation of DLC films induces the formation of sp2 bonds, which release the internal stress, the release effect appears to be not so significant for DLC.8
Recently, ultrananocrystalline diamond (UNCD)/hydrogenated amorphous carbon (a-C:H) composite (UNCD/a-C:H) films comprising diamond grains with diameters of 2–10 nm have attracted researchers owing to their unique physical properties such as thermally stable up to 550°C, a low friction coefficient, and extremely smooth surface. UNCD/a-C:H films have been mainly prepared by chemical vapor deposition (CVD), wherein hydrogen has an important role in the film growth.9,10 Although UNCD/a-C:H films can be grown even in an atmosphere without the inflow of hydrogen gas, plasma in CVD is comprised of atomic hydrogen and hydrocarbon radicals and the resultant films contain hydrogen.11
Whereas UNCD/a-C:H films have been mainly prepared by CVD method, several years ago, our group successfully prepared UNCD/a-C:H films in a hydrogen atmosphere, UNCD/nonhydrogenated nitrogenized amorphous carbon (a-C:H:N) composite (UNCD/a-C:N) films in a nitrogen atmosphere, and UNCD/hydrogenated nitrogenized amorphous carbon (a-C:H:N) composite (UNCD/a-C:H:N) films in a nitrogen and hydrogen mixed gas atmosphere, by pulsed laser deposition (PLD), as they have been reported previously.12–18 PLD is a kind of physical vapor deposition (PVD), and the formation of UNCD grains by PLD might be attributable to the supply of highly energetic carbon species such as C+ ions and C atoms at a high density onto a substrate,19 and its mechanism should be distinctively different from that in CVD. The formation by PLD need not the pretreatment of substrates with diamond powder, in other words, the nucleation of diamond spontaneously occurs during the deposition.
The deposition of DLC and nanocrystalline diamond films on metals has received attention from the viewpoints of engineering application. For example, for the deposition on titanium that is a promising candidate applicable to aerospace, biomedical, and chemical plant, however whose large friction coefficient is a bottleneck for their applications, the coating of titanium with carbon films possessing low friction coefficients can improve the large friction of titanium. It is very difficult for hard carbon films to be deposited on titanium, because the thermal expansion coefficients of carbon films are extremely small whereas those of metals are oppositely large, which results in a large residual internal stress in the films at room temperature. In the case of the stress being large, the films are spontaneously peeled from the titanium.
Thus far, several research groups have deposited nanocrystalline diamond films on titanium substrates by CVD, wherein the substrate temperature for the growth is moderately set in order to reduce an internal stress in the films.20,21 By PLD, UNCD/a-C:H films are grown at a substrate temperature of 550°C, which might be at least 50°C lower than that in CVD of the nanocrystalline diamond films on titanium.20,21 However, it is difficult for UNCD/a-C:H films to be directly deposited on titanium substrates by PLD, in other word, the films are easily peeled from the substrates in the initial stage of deposition. Since the growth mechanism of UNCD/a-C:H films by PLD differs from that by CVD films, the internal stress in the deposited films is not determined only by the substrate temperature.
In this work, UNCD/a-C:N and UNCD/a-C:H:N films were deposited by PLD, and we studied the nitrogenation effects on adhesion between the films and titanium substrates. We report that the adhesions of films to titanium substrates are obviously improved due to the nitrogenation that excludes hydrogenation. In addition, we discuss the origins of the nitrogenation effects from the chemical bonding structural viewpoints on the basis of the spectroscopic measurements with synchrotron radiation.
Experimental
Approximately 100 nm UNCD/a-C:N and UNCD/a-C:H:N films were deposited on polished titanium substrates at a substrate temperature of 550°C at a nitrogen pressure of 53.3 Pa by PLD using a graphite target. The chamber was evacuated down to a base pressure of less than 10−4 Pa, and the ambient nitrogen pressure during the film deposition was set to be 53.3 Pa at a nitrogen flow rate of 3 sccm for the preparation of UNCD/a-C:N films. For comparison, UNCD/a-C:H:N films were prepared in a nitrogen and hydrogen mixed gas atmosphere at nitrogen and hydrogen gas inflow rates of 3 and 5 sccm, respectively. Unlike CVD methods that requires the pretreatment of substrates with diamond powder, PLD need not such a seeding procedure. Prior to the deposition, titanium substrates were cleaned by acetone and methanol using ultrasonic method. The distance between the substrate and target was fixed to be 15 mm. An ArF excimer laser beam with a wavelength of 193 nm and full-width at half-maximum of 20 ns was employed for ablation. The energy and repetition rate of pulsed laser beams was 100 mJ and 50 Hz. The laser beam was focused onto a rotating graphite target using a spherical lens at an angel of 45°. The irradiation area on the target was approximately 2 mm2 and the irradiance was 1.8 × 108 W/cm2.
The existence of UNCD grains was examined by powder X-ray diffraction (XRD) with 12 keV X-rays from synchrotron radiation at beamline 15 of the Kyushu Synchrotron Light Research Center/SAGA Light Source. For its measurement, the film peeled from the substrate was powdered and placed in a capillary with a diameter of 0.3 mm, and Debye-Scherrer rings were recorded on imaging plates. The details were reported in our previous work.15,22 In addition, the debris of films peeled from the substrates were observed by transmission electron microscopy (TEM). The chemical bonding structures of the films were evaluated by X-ray photoemission and near-edge X-ray absorption fine-structure (NEXAFS) spectroscopies using synchrotron radiation at beamline 12 of the Kyushu Synchrotron Light Research Center/SAGA Light Source. For the X-ray photoemission spectroscopic measurement for estimating the chemical composition of films, a MgKα line (1253.6 eV) was also employed as an incident light source, in addition to 350 eV light from synchrotron radiation for investigating the chemical bonding structures. The chemical composition in the film depth direction was measured by energy dispersive X-ray (EDX) spectroscopy. All the X-ray photoemission spectra were measured at an emission angle of 90° to the film surface and room-temperature. Argon ion bombardment etching was not carried out in order to avoid the destruction of diamond grains and a change in the chemical bonding structure near the film surface.23 The intensity of NEXAFS spectra was calibrated with an incident photon flux and the measurement was made in total electron yield (TEY) mode by measuring the current from the sample to ground. Highly oriented pyrolytic graphite (HOPG) was used as a reference sample for energy calibration and comparison.
Results and Discussion
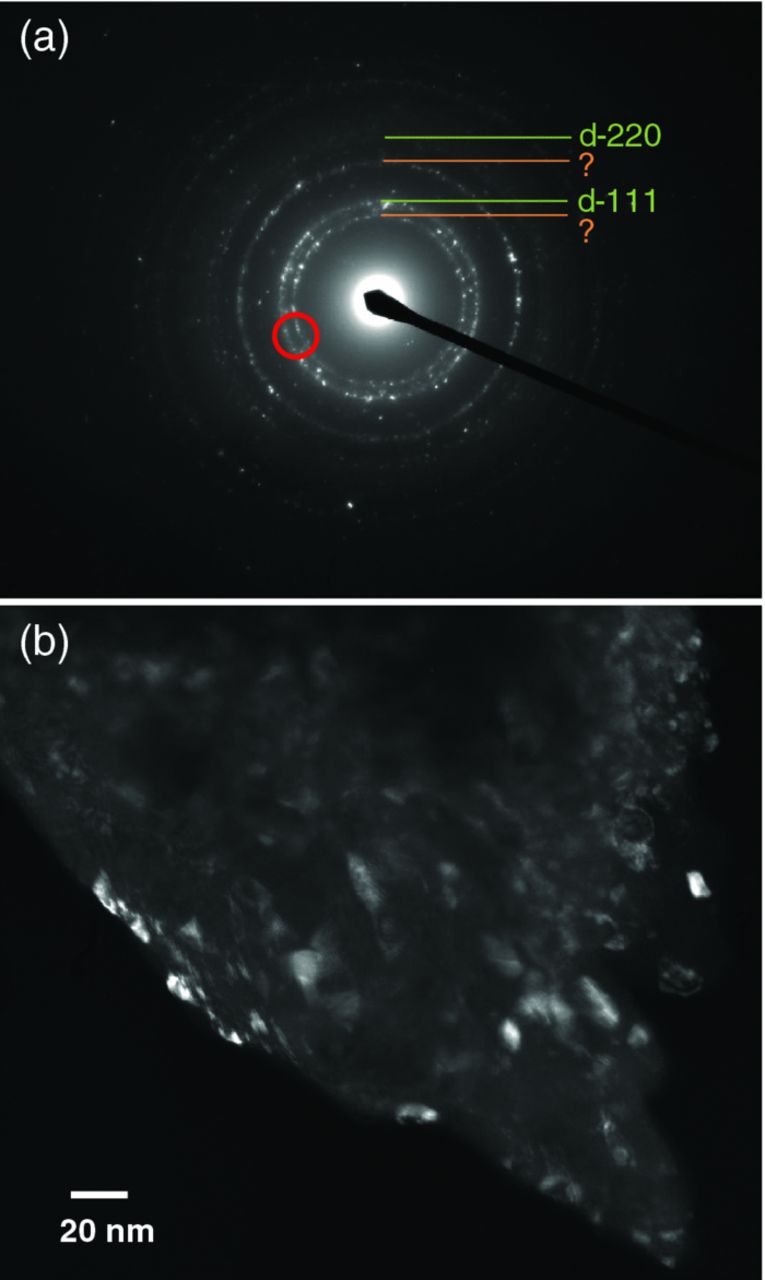
The existence of UNCD grain was confirmed from the XRD powder measurement. The grain size was estimated to be approximately 8.5 nm and 2.5 nm from diamond 111 peaks for UNCD/a-C:N and UNCD/a-C:H:N films, respectively, as reported in our previous works.15,22 Figure 1a shows the electron diffraction (ED) pattern of UNCD/a-C:N film. Since UNCD grains are easily destroyed and changed to amorphous carbon, by ion bombardment, the sample for this measurement is the debris of film peeled from the substrate and it is not processed. The ED pattern exhibits diffraction rings due to diamond 111 and 220 and additional unknown rings, whereas the UNCD/a-C:H:N film exhibits diffraction rings due to diamond. In the UNCD/a-C:N film, grains of unknown phase and diamond coexist. The unknown rings might be attributable to some carbon nitride or distorted diamond. We could not find carbon nitride phases assignable to the unknown rings. The assignment requires additional structural analysis. The dark field TEM image, which was taken with the partial diffraction beam indicated in red in Fig. 1a, certainly shows the existence of diamond grains in the debris.
Figure 1. (a) ED pattern of UNCD/a-C:N film and (b) dark-field TEM image taken with partial direction beam indicated in red circle in (a).
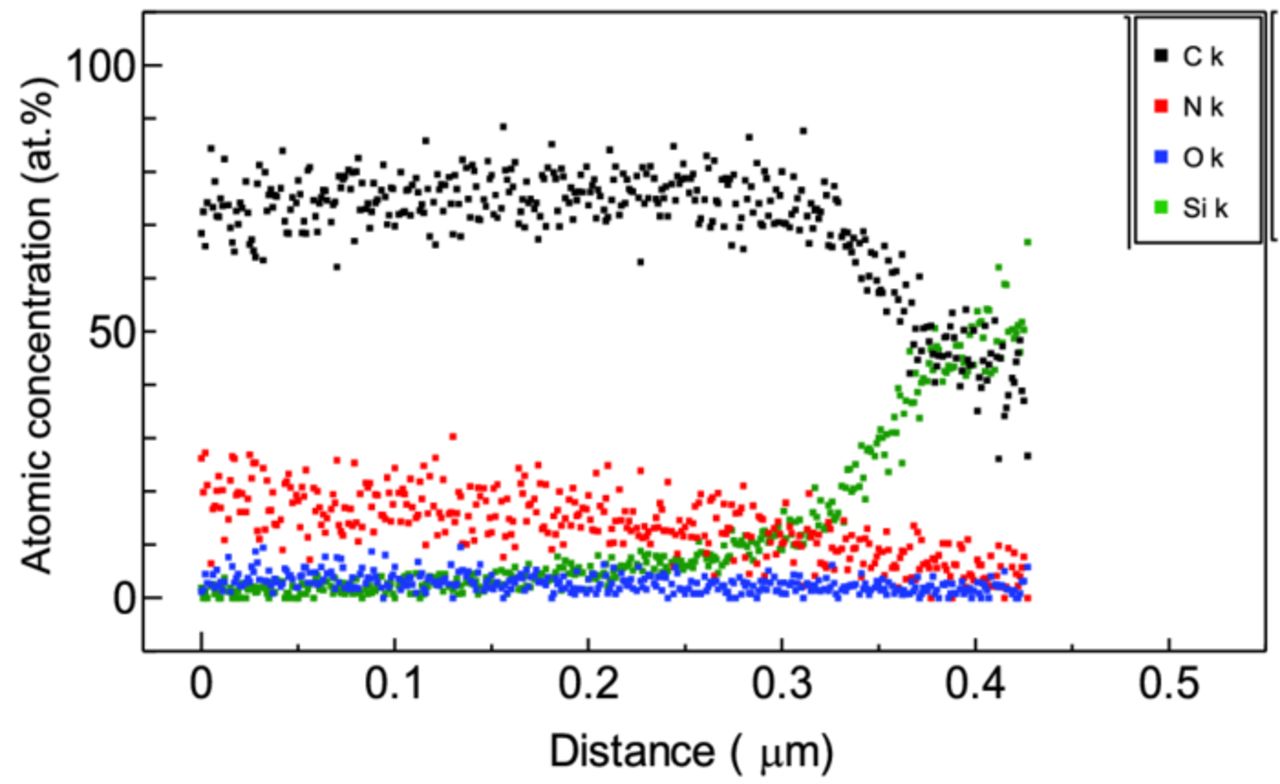
We have already reported that the film quality is uniform in the film depth direction on the basis of the cross-sectional TEM observation of films.12 The growth of UNCD/a-C:H films by PLD is, so to speak, the accumulation of repeated nucleation in pulsed deposition processes. Thus, the deposition need not the pretreatment of substrates with diamond powder and the resultant films have no distribution of the quality in the depth direction. Figure 2 shows the depth profile in the chemical composition of a UNCD/a-C:H:N film deposited on a Si subatrate, measured by EDX spectroscopy. The chemical composition does not have an evident distribution in the film depth direction. The similar results has been obtained also from the X-ray photoemission spectroscopy combined with Ar ion bombardment etching.
Figure 2. Depth profile in the chemical composition of UNCD/a-C:H:N film deposited on Si substrate, measured by EDX spectroscopy.
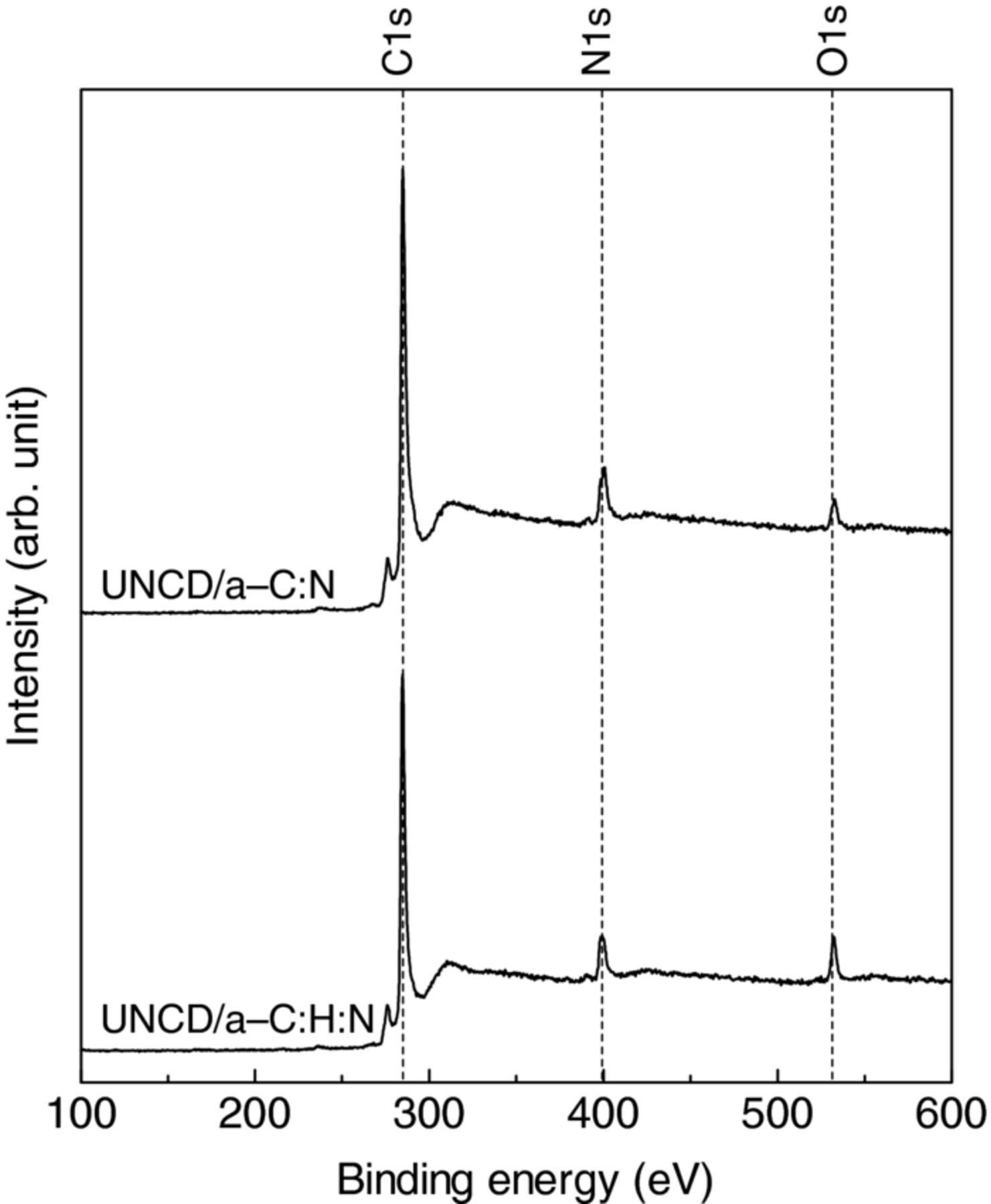
The X-ray photoemission spectra of UNCD/a-C:N and UNCD/a-C:H:N films, which were measured in a wide range, are shown in Fig. 3. A N 1s peak is clearly observed, which evidently indicates the incorporation of nitrogen atoms into the deposited film from the nitrogen atmosphere. The nitrogen atomic fraction (fN) was calculated in the same manner as that in our previous work,17 using the following equation:
![Equation ([1])](https://content.cld.iop.org/journals/2162-8777/2/11/M33/revision1/jss_2_11_M33eqn1.jpg)
Here, AN, SN, and ∑ Ai/Si are the area of N 1s peak, atomic sensitivity factor of nitrogen, and summation of the ratios between peak areas and atomic sensitivity factors, respectively. The values of atomic sensitivity factors for C 1s, N 1s, and O 1s are 1.00, 1.77, and 2.85.24,25 The O 1s peak disappears immediately for argon ion bombardment. Thus, the origin of the oxygen peak does not come from the bulk of film. The nitrogen atomic concentration was estimated to be 9.7 at% and 9.9 at% for the UNCD/a-C:N and UNCD/a-C:H:N films, respectively. Even if the contribution of the oxygen peak to the estimation is considered, the estimated nitrogen concentration was 9.5 and 9.4 at% for the UNCD/a-C:N and UNCD/a-C:H:N films, respectively. The existence of the oxygen peak hardly affect the estimation of nitrogen atomic concentration.
Figure 3. X-ray photoemission spectra of UNCD/a-C:N and UNCD/a-C:H:N films on titanium substrates, measured with MgKα line (1253.6 eV).
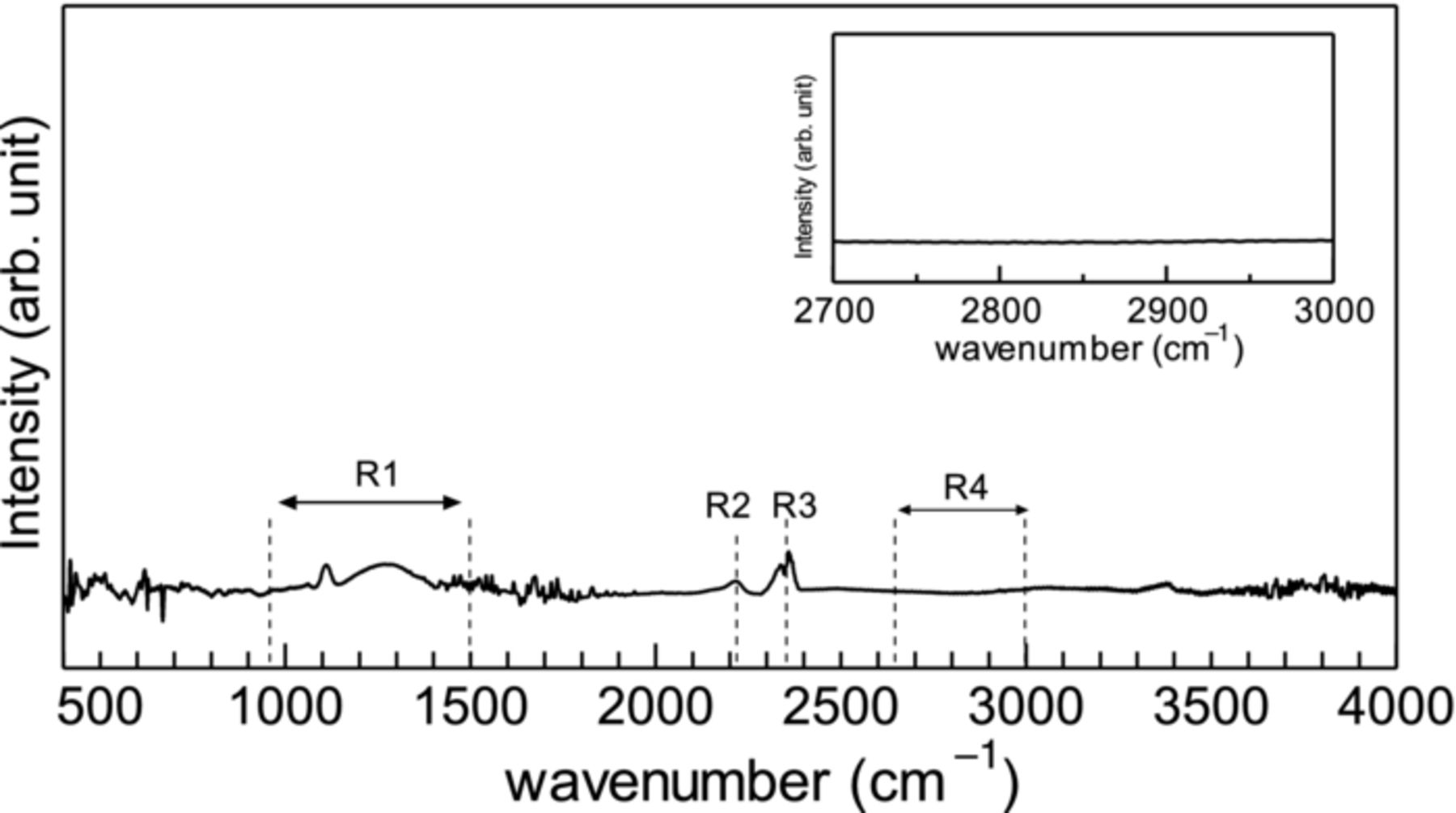
Figure 4 shows the Fourier transform infrared (FTIR) spectrum of UNCD/a-C:N film. The R1 range spectrum is due to C=N, C=C, and C−N. The R2 and R3 range spectra are due to C≡C and CO2, respectively. A spectrum due to CHn is not observed in the R4 range at all, which indicates that the UNCD/a-C:N film does not contain hydrogen as naturally-expected.
Figure 4. FTIR spectrum of UNCD/a-C:N film. The inset is the magnification of R4 wavenumber range.
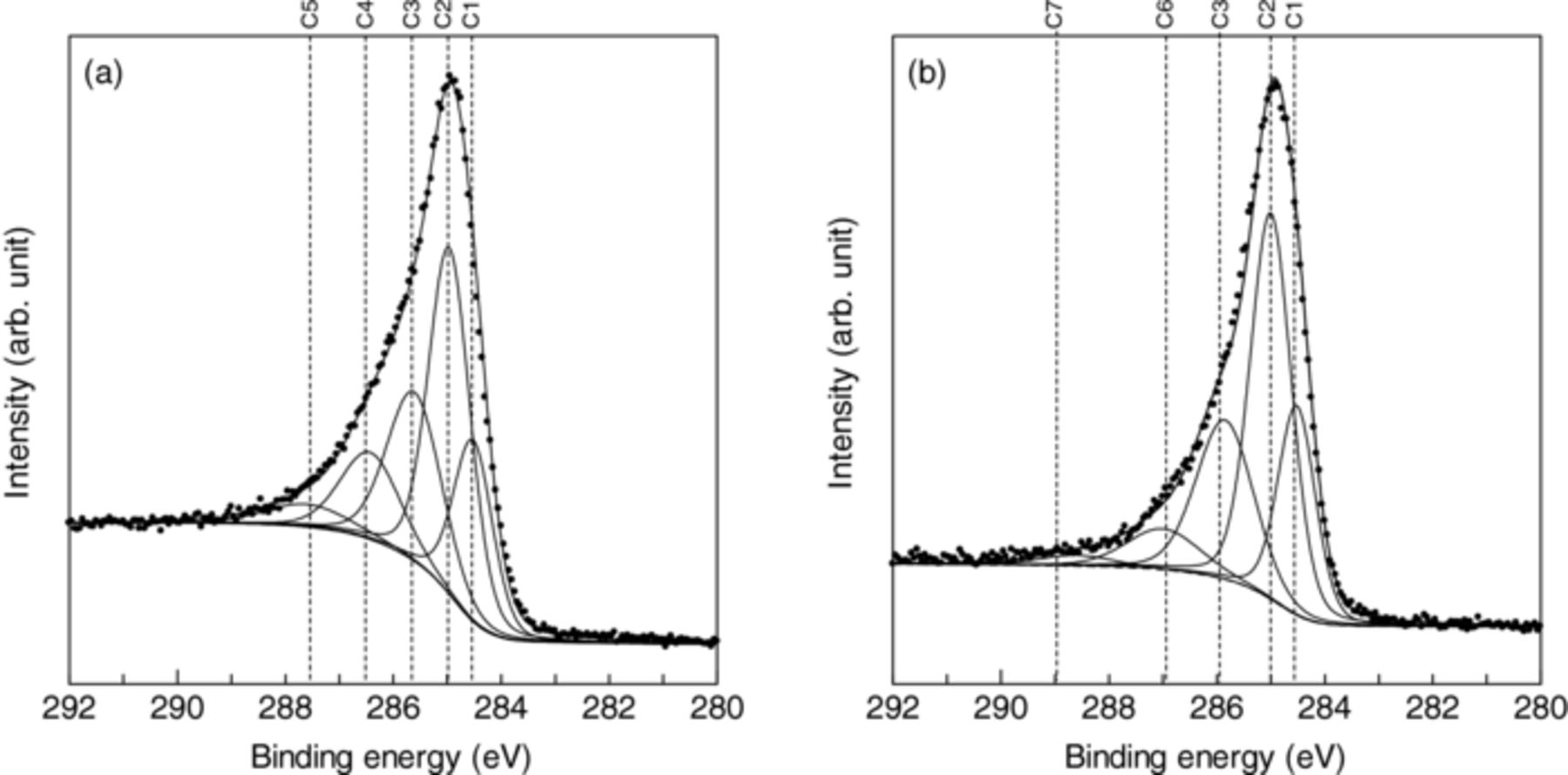
C 1s X-ray photoemission spectra measured with 350 eV X-ray from synchrotron radiation were decomposed into several components with Gaussian (80%)/Lorentizian (20%) functions17 after the backgrounds were subtracted by Shirley's method.26 In addition to the binding energy of spectra being sensitively influenced by the chemical bonding environment,27 a charged sample surface also cause an energy shift, namely false chemical shift.28 Thus, the effects of charge up on the measurement were carefully checked by comparing with the HOPG spectrum.
The spectrum of the UNCD/a-C:N film were decomposed into several component spectra. The components C1, C2, C3, C4, and C5 are centered at 284.5, 285.0, 285.6, 286.4, and 287.6 eV, respectively, as shown in Fig. 5a. The C1 and C2 components are due to C=C (sp2) and (C–C) (sp3) bonding, respectively.18 The C5 component is probably due to C=O bonding,29 which is resultant from the exposure of the film to air. The C3 component is assigned to be due to C=N bonding,30 which indicates incorporated nitrogen atoms certainly form the bonds with carbon. The assignment of the C4 component might be due to C–N and/or C≡N bonds. In the range between 286 and 287 eV, components assigned to C–N and C≡N bonds were reported for polymers and carbon nitride materials,31–33 and their binding energies are very close to each other. It has been reported that at a large nitrogen concentration nitrile-like structure mainly comprising C≡N bonds is formed and its amount increases with the nitrogen concentration.34 Since the nitrogen concentration of 9.7 at.% is relatively large, C≡N bonds are expected to be formed. Figure. 5b shows the X-ray photoemission spectrum of UNCD/a-C:H:N film. It contains a well resolved component C6 centered at 287.0 eV and it might be predominantly attributable to C–N bonds. The component C7 is assigned to COOH bonds.
Figure 5. C 1s X-ray photoemission spectra of (a) UNCD/a-C:N and (b) UNCD/a-C:H:N films, measured with a 350 eV incident X-ray from synchrotron radiation.
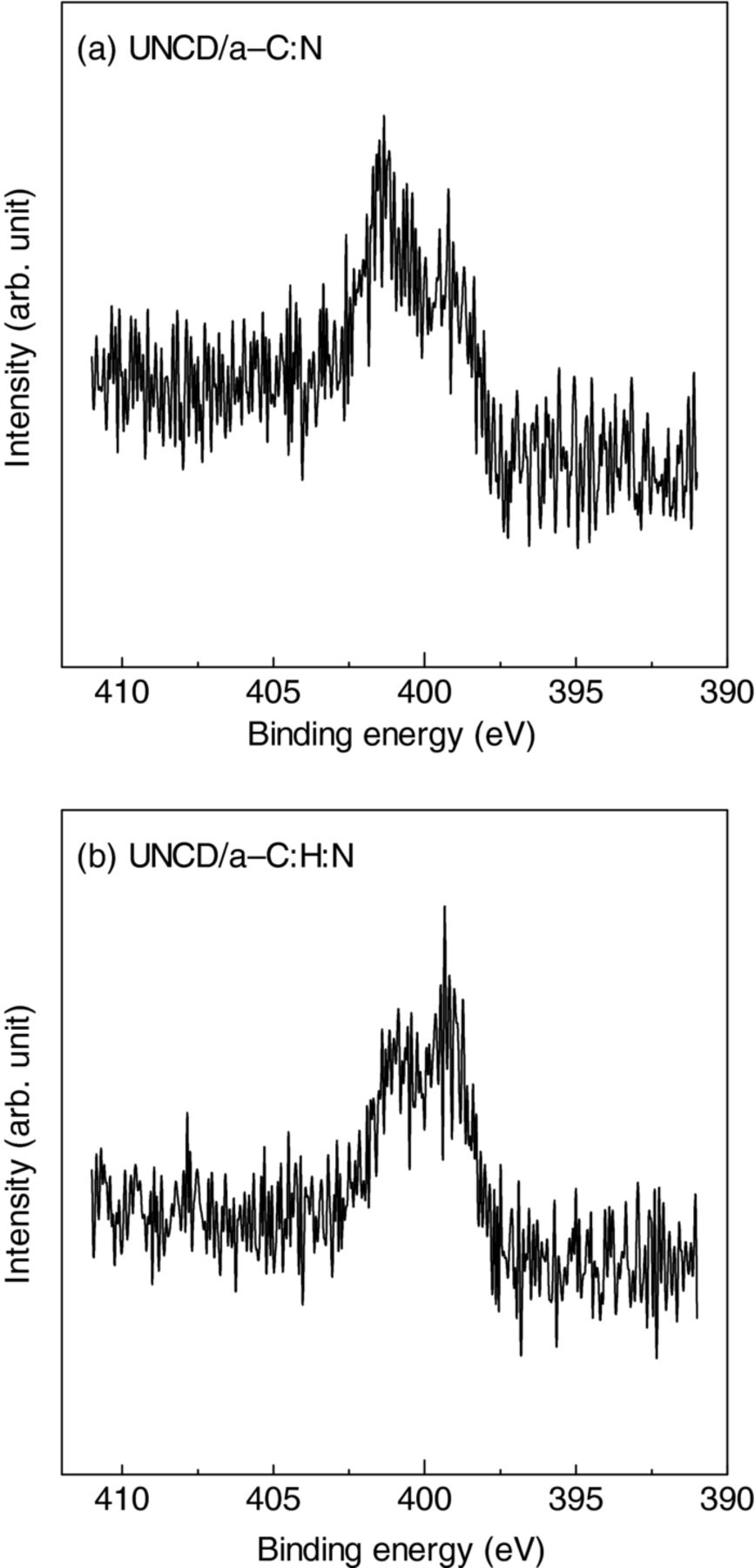
The N 1s X-ray photoemission spectra of UNCD/a-C:N and UNCD/a-C:H:N films are shown in Fig. 6. The N 1s spectra have two peaks centered at 398.7 and 400.17 eV and they are assigned to C–N and/or C≡N and C=N bonds, respectively. The UNCD/a-C:N film exhibits the intense 398.7 eV peak compared with that in the spectrum of UNCD/a-C:H:N film, which is perhaps attributable to the existence of C≡N bonds.
Figure 6. N 1s X-ray photoemission spectra of (a) UNCD/a-C:N and (b) UNCD/a-C:H:N films, measured with a 350 eV incident X-ray from synchrotron radiation.
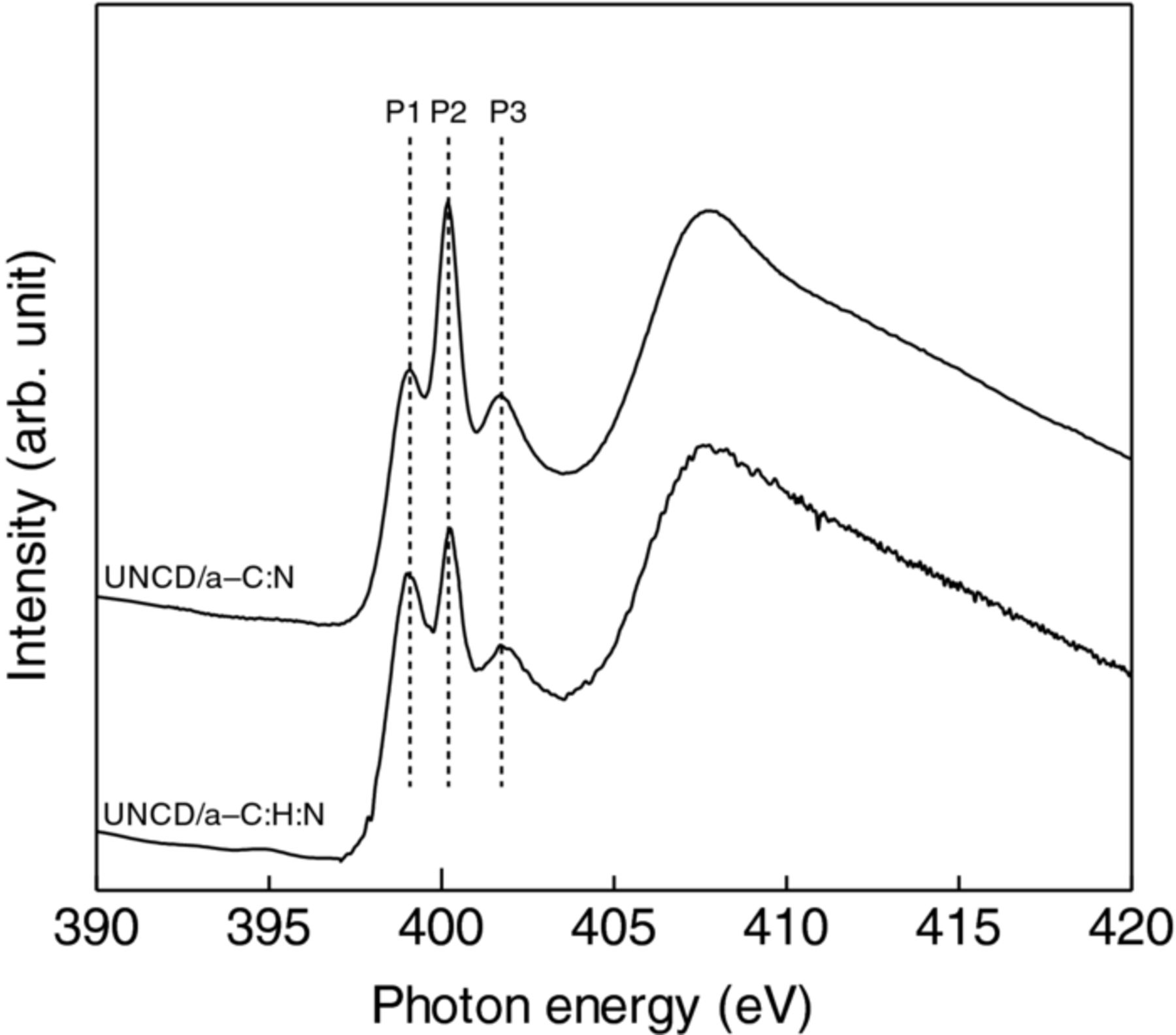
NEXAFS, in a which core electrons is excited to an unoccupied state, is a sensitive method to probe local chemical and electronic structures of atoms. For carbon nitride materials, N K-edge NEXAFS spectra have received much attention since they are normally composed of three distinctive components.35–41 Figure 7 shows the N K-edge NEXAFS spectra of UNCD/a-C:H:N and UNCD/a-C:N films. The relative intensity of each peak might represent a clue to know chemical bonding environments of nitrogen and carbon atoms. The P1 peak is probably due to the C=N bonds from previous reports.42,43 The P3 peak is probably due to substitutionally incorporated nitrogen atoms that form aromatic rings44 or three-fold nitrogen in a graphitic network.45,46
Figure 7. N K-edge NEXAFS spectra of UNCD/a-C:N and UNCD/a-C:H:N films.
The P2 peak is the most important peak that receives much attraction from many scientists, since its appearance depends on the structure of nitride materials. The P2 peak might be predominantly attributable to C≡N bonds from the following reasons: (a) a theoretical calculation made by N. Hellgren et al.37 predicts that a peak at 399.5 eV is sharpened due to the formation of nitrile structure; (b) in comparison with a spectrum reported by Cameron et al., the appearance of this peak coincides with the formation of C≡N bonds that mainly construct polyacrylonitrile (PAN) structure;47 (c) it is well known that nitrile is preferentially formed at a large nitrogen concentration, and thus 9.7 at.% nitrogen-incorporated film in this study probably comprise C≡N bonds accompanied by the formation of nitrile. The P2 peak in the UNCD/a-C:N film spectrum is intense as compared with that in the UNCD/a-C:H:N film spectrum. This implies that the UNCD/a-C:N film more probably comprises C≡N bonds, which is not in contradiction with the X-ray photoemission results.
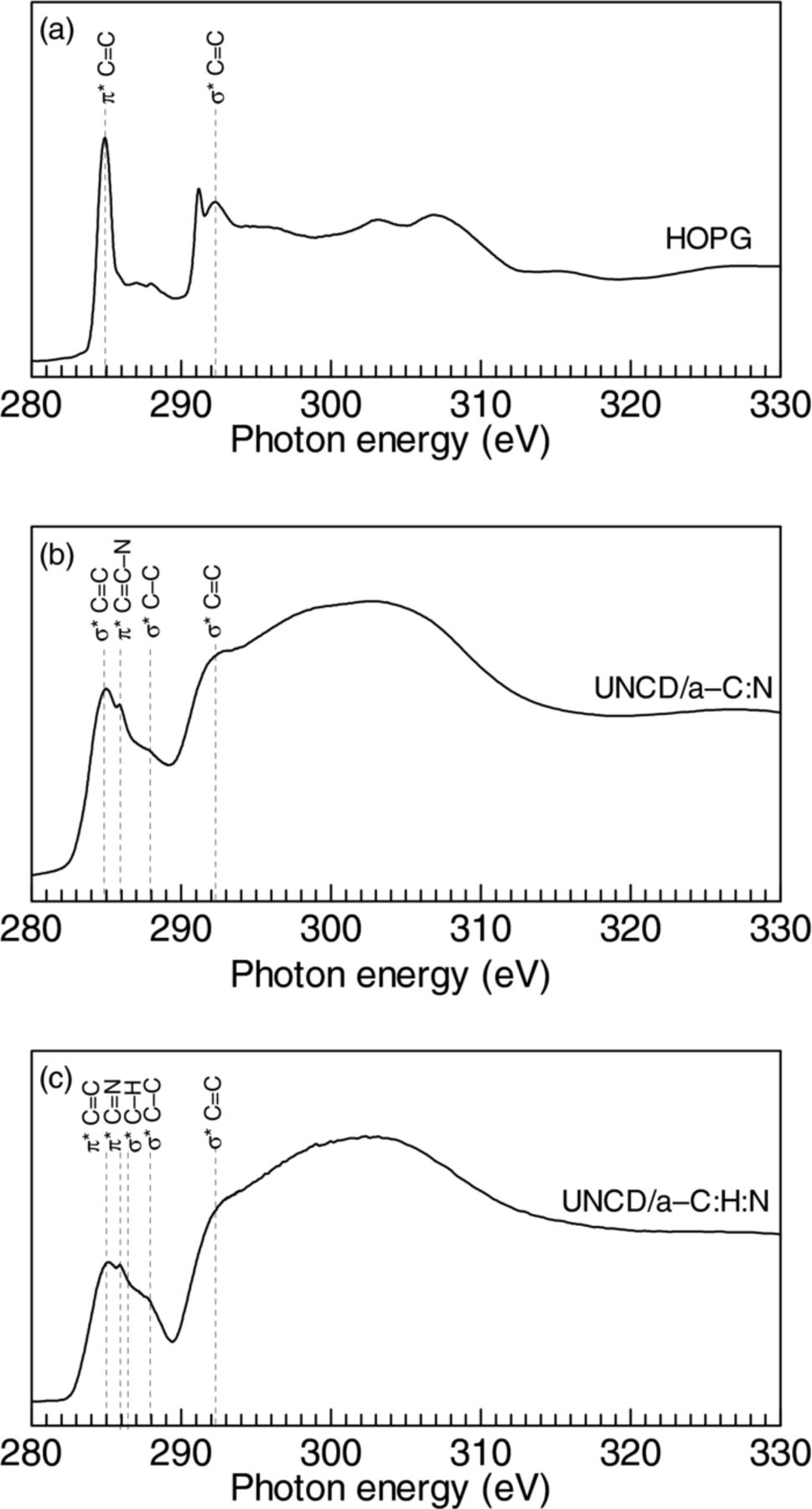
The C K-edge NEXAFS spectra of (a) HOPG for comparison, (b) UNCD/a-C:N, and (c) UNCD/a-C:H:N films are shown in Fig. 8. The HOPG spectrum shows a well-known signatures of π* states at 285 eV and σ* states at 292 eV, respectively. Since HOPG has an anisotropic structure, the HOPG spectrum is strongly affected by an incident angle of X-ray:48–50 π* peak becomes minimal for incident X-ray perpendicular to π* orbital, and on the other hand, the peak strengthens for X-ray parallel to the π* orbital.48 In this measurement, HOPG was measured at an incidence angle of 45°. For both films, UNCD grains in the films are nonoriented, and thus the incidence angle does not influence the spectral profile. The HOPG spectrum shows two clear peaks due to π* C=C and σ* C=C bonds at 285.0 eV and 292.5 eV, respectively.
Figure 8. C K-Edge NEXAFS spectra of (a) HOPG, (b) UNCD/a-C:N and UNCD/a-C:H:N films.
The UNCD/a-C:N film shows distinctive characteristics as follows. A peak at 285 eV and peak at 292.1 eV are attributable to π* C=C and σ* C=C bonds, respectively, according to previous reports.16 A peak at 288.4 eV is attributable to σ* C–C bonds. The existence of UNCD grains is expected to partially contribute to this peak.
A peak at 285.5 ∼ 287.0 eV is a puzzler for assignment, and it might originate from the nitrogenation of the film. From the peak position, the candidates are π*C=C–N,51 π*C=N,13,16,35,43,52 and C≡N bonds. The above-mentioned X-ray photoemission spectroscopic results indicated a possibility of the PAN formation53 with a structure of (C3H3N)n comprising C≡N bonds. Since it is reported that the C K-edge NEXAFS spectrum of PAN structure exhibits a sharp π*C≡N transition at 287 eV,43 the assignment to C≡N bond is most likely. As another possibility, the formation of N–C≡N or C=N=N bonds were reported for hydrogen-free carbon nitride.54 Our film is structurally similar except for the existence of UNCD grains, and thus the assignment to π*C=N is also probable, which is also consistent with the X-ray photoemission spectroscopic results. For the UNCD/a-C:H:N, as shown in Fig. 6c, the π*C=C peak is obviously broad with a shoulder at 286 eV.16 This is perhaps due to the existence of π*C=N bonds. From the appearance of σ* C−H peak, hydrogen incorporation was confirmed.16
Conclusions
UNCD/a-C:N films with a nitrogen content of 9.7 at.% and UNCD/a-C:H:N films with a nitrogen content of 9.9 at.% were deposited on titanium substrates by PLD with graphite targets. From the chemical bonding structural analysis by NEXAFS and X-ray photoemission spectroscopies, it was found that the formation of C=C, C≡C, C=N, and C≡N bonds is specific to the UNCD/a-C:N films. These formations among predominant sp3 bonding configuration including UNCD grains is expected to locally soften the film, which might result in a release of an internal stress in the film. The specific to UNCD/a-C:N films is containing a large number of grain boundaries (GBs) inside the films. Here, GBs exactly denote the interfaces between UNCD grains and those between UNCD grains and an a-C matrix. It is well known that incorporated foreign elements are preferentially located in grain boundaries. Thus, C=C, C≡C, C=N, and C≡N bonds might be preferentially formed at GBs, and its formation might effectively release an internal stress in the films. UNCD/a-C:N is a unique candidate material for coating and also is applicable to a buffer layers for depositing diamond and nanodiamond films on metal substrates.
Acknowledgments
This work was partially supported by "Nanotechnology Network Japan" (Kyushu Synchrotron Light Research Center, Proposal Nos. 081152N, 090423N) and "Open Advanced Research Facilities Initiative" (Kyushu Synchrotron Light Research Center, Proposal Nos. 100320AS and 1104035AS) of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, Advanced Low Carbon Technology Research and Development Program (ALCA), Japan Science and Technology Agency (JST), and a research grant from the Mazda Foundation. The authors would like to express their deep gratitude to Dr. Hiroyuki Setoyama, and Dr. Eiichi Kobayashi for their professional support in both the X-ray photoemission and NEXAFS measurements.