Abstract
In order to develop the suitable Cu electrolyte for TSV filling using period pulse reverse (PPR) electroplating, the operating mechanism of reverse pulse on the adsorption of additives within TSV was systematically investigated. Whether the promotion or reduction of the adsorption of polyethylene glycol (PEG), bis (sodiumsulfopropyl) disulfide (SPS) and Janus Green B (JGB) by reverse pulse was determined by the charge of the formed complex of this additive with Cu+ and Cl−. The charge of formed complex was dependent on the Cl− concentration. The reverse pulse had no significant effect on the adsorption of single JGB. In comparison, the composite JGB-PEG inhibitor could be repelled by reverse pulse at the microvia bottom. For the composite PEG-SPS or PEG-JGB-SPS, since the preferentially adsorbed Cu+-Cl−-PEG dense layer could effectively block the transportation of Cl−, few sites of Cl− were left for the SPS adsorption and then mainly formed positively charged SPS-Cu+, which accounted for the detachment of SPS by anodic pulse current at microvia entrance in the presence of PEG. Based on the change of the additives coverage surface by reverse pulse, the microvia filling performances in PPR plating compared to those in DC plating could be well explained.
Export citation and abstract BibTeX RIS

This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 License (CC BY, http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse of the work in any medium, provided the original work is properly cited.
Three-dimensional integrated circuitry (3D-IC) with through Si via (TSV), is a promising technology to obtain vertical electric interconnection, high packaging density, faster signal transmission, and lower power consumption.1,2 The Cu filling by electroplating is one of the core and critical procedures in TSV fabrication. However, it is a great challenge in Cu electroplating to provide a void-free filling for the TSV with high aspect ratio (AR). The "super-filling" was widely used to obtain void-free filling for micro-sized blind via, i.e., the growth at micro-sized via or trench opening was limited and the growth at the bottom was accelerated, which could be enabled by special additives combination in Cu electrolyte.3–9 Several operating mechanisms of additives adsorption within microvia have been invoked to explain this super-filling process, such as the curvature enhanced accelerator coverage (CEAC) model,3–5 the convection-dependent adsorption (CDA) model6,7 and time-dependent transport-adsorption mode.8,9 The commonly used additives can be divided into three categories: accelerator, such as bis (sodiumsulfopropyl) disulfide (SPS), inhibitor, such as polyethylene glycol (PEG), and leveler, such as Janus Green B (JGB). In addition, the chloride ion also played an essential role in Cu electrolyte to promote the additive adsorption, mass transport and Cu ion reaction.10–13
Another available approach for promoting void-free filling was the use of period pulse reverse (PPR) plating.14 Reverse pulse was an effective approach to mitigate the Cu ion depletion at the microvia bottom.15,16 In the previous studies, Hong et al.17 reported that an enhanced filling result was obtained by 3-step PPR using one additive. Sun et al.18 reported that the PPR plating was effective to remove those seams and voids at the microvia centers in an electrolyte with JGB additive. However, Hofmann et al.19 reported that the PPR plating with 2-additives produced a poorer filling result than that without additives. Therefore, the difficulty of PPR plating for TSV filling is the application of additive. It appeared that the additive formula in direct current (DC) plating process may be unsuitable in PPR plating because the influence of reverse pulse on the additive adsorption. However, extremely few reports focused on the effect of reverse pulse on additives adsorption within the microvia of TSV. Hayase et al.20,21 reported that the reverse pulse could remove accelerator from copper surface with strong agitation and was helpful for a bottom-up filling of TSV using a Cu electrolyte with PEG-JGB-SPS additive system. Kim et al.22,23 reported that the anodic step promoted the displacement of PEG-Cl− by SPS at the early stage of electrodeposition and improved both superfilling and leveling performances. However, up to date, the operating mechanism of reverse pulse on the additive adsorption within the microvia is still unclear.
In this work, we intended to systemically investigate the operating mechanism of reverse pulse on the additives adsorption within the TSV microvia. The Cu filling by PPR electroplating and electrochemical analysis were carried out to investigate the effect of reverse pulse on the adsorption behavior of the single suppressor, accelerator, leveler and composite additives. Based on that, the related operating mechanisms of reverse pulse on the additives adsorption within the microvia were built. The study result was expected to provide theoretical guidance to develop the suitable Cu electrolyte for TSV filling using PPR electroplating.
Experimental
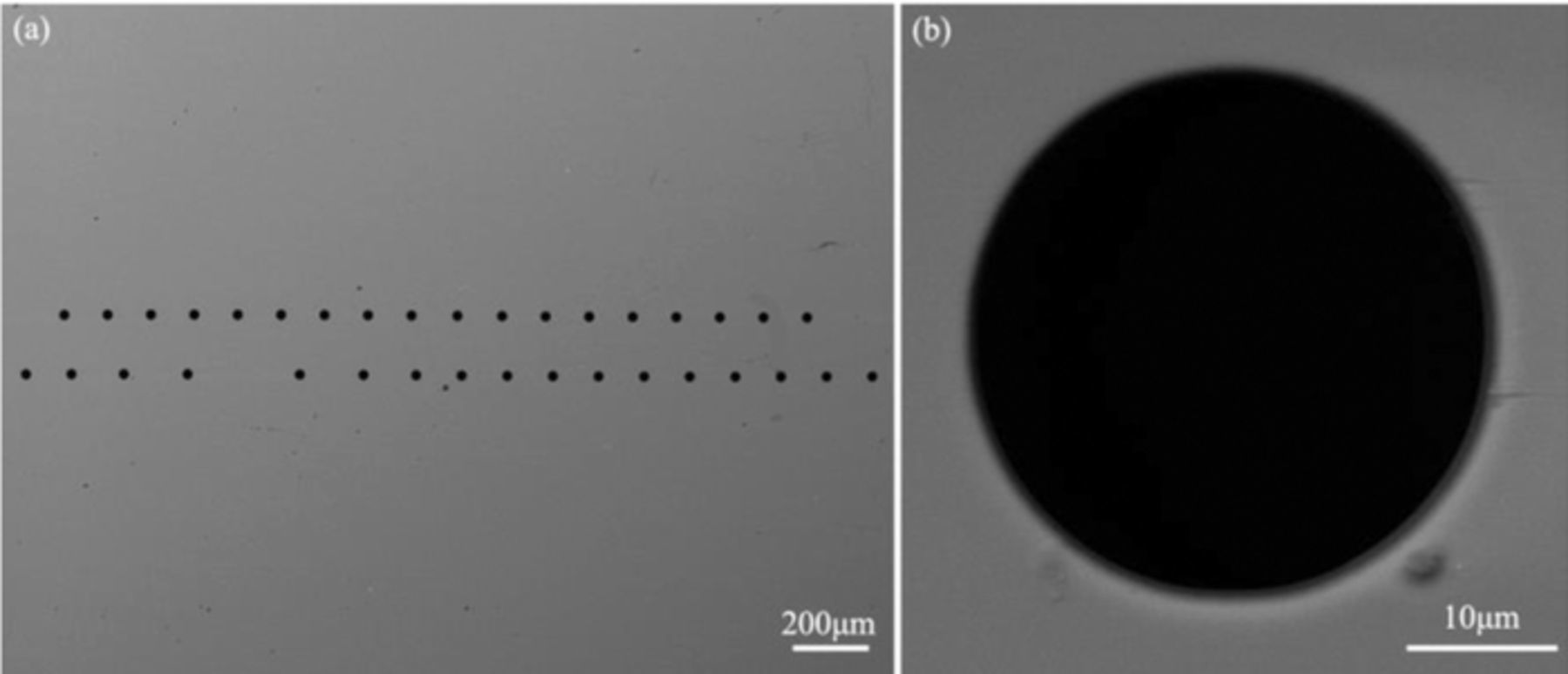
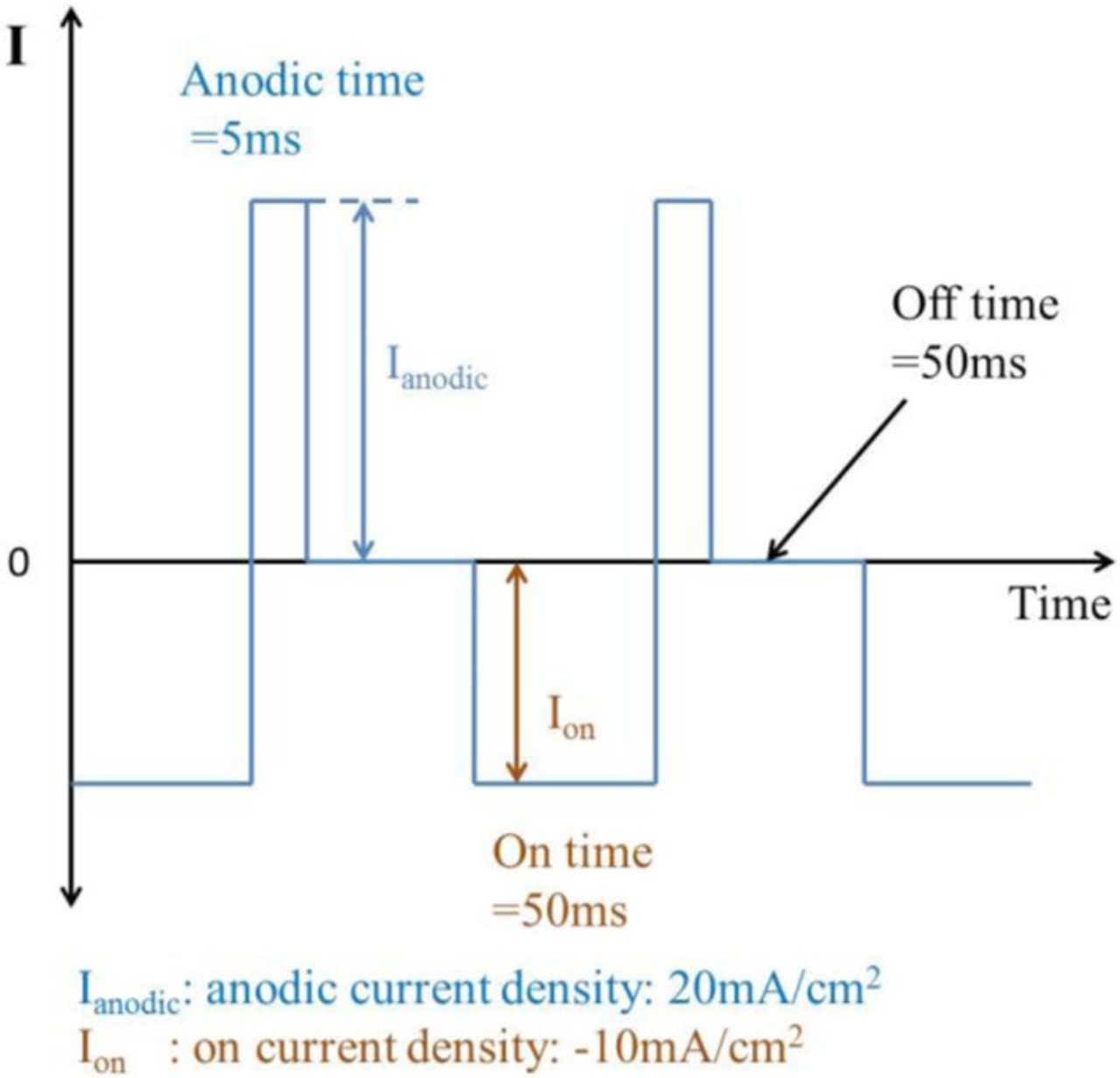
Blind micro-sized vias in 8-inch wafer were fabricated by lithography and deep reactive ion etching (DRIE) process. The blind microvia had an aspect ratio of 100μm/30μm (depth/diameter). The sidewall of microvia was firstly coated by the SiO2 insulating layer with a thickness of 500 nm by a thermal oxidation process. Then a Ti adhesion layer with a thickness of 20 nm and a copper seed layer with a thickness of 150nm were fabricated by CVD process. The unit chip sample for Cu electroplating was shown in Fig. 1. Prior to the electroplating filling, the sample was treated in a vacuum process in Cu electrolyte, so that the bottom of microvia can be fully wetted. The basic Cu electrolyte was composed of 50 g/L CuSO4, 200 g/L H2SO4 and 60 mg/L Cl−. The applied additives in electrolytes containing single additive were 200 ppm PEG, 10 ppm JGB and 10 ppm SPS respectively. The additives combinations in composite electrolytes were 200 ppm PEG+10 ppm JGB, 200 ppm PEG+10 ppm SPS and 200 ppm PEG+10 ppm SPS+10 ppm JGB respectively. During electroplating, a 0.05% P content copper plate was used as the anode and the distance between the electrodes was 100 mm. The solution was stirred by a magnet bar with a rotation rate of 200 rpm and the temperature was room temperature. A commercial electrochemical workstation (PGSTAT204, Auto-Lab) was used as a DC and PPR power supply. The applied current density in DC plating was −10 mA/cm2. In PPR electroplating, the PPR waveform was consisted of a forward deposition stage with a relatively low current density of 10 mA/cm2, an anodic pulse with a relative high current density of 20 mA/cm2, and a pause stage with a time of 50 ms, as shown in Fig. 2. After careful polishing process, the cross-sections of the filled microvia were observed using field emission scanning electron microscopy (FE-SEM) to determine the filling qualities. Also, the Cu grain morphology was investigated using electron backscattered diffraction (EBSD) method.
Figure 1. Top view of the applied TSV chip for Cu electroplating.
Figure 2. The applied current waveform in PPR plating process.
The electrochemical analysis was carried out using an electrochemical working station (PGSTAT204, Auto-Lab) equipped with a rotating disk electrode (RDE, ALS). A platinum disk, a platinum foil and a Ag/AgCl electrode (3mol/L NaCl electrolyte) were used as the working electrode, counter electrode and reference electrode respectively. The rotation disk speeds of the RDE were set to be 0, 100 and 1000 rpm respectively, which were used to simulate the mass transfer environment at the relatively shallower and deeper location of the microvia respectively. In chronopotentiometry test, a constant deposition current was applied, followed by a short-time anodic current, which was used to simulate the reverse pulse in PPR plating process. The potential variation after anodic current was used to reflect the adsorption or desorption behavior of additives within the microvia.
Results and Discussion
Effect of reverse pulse on microvia filling performance using single additive
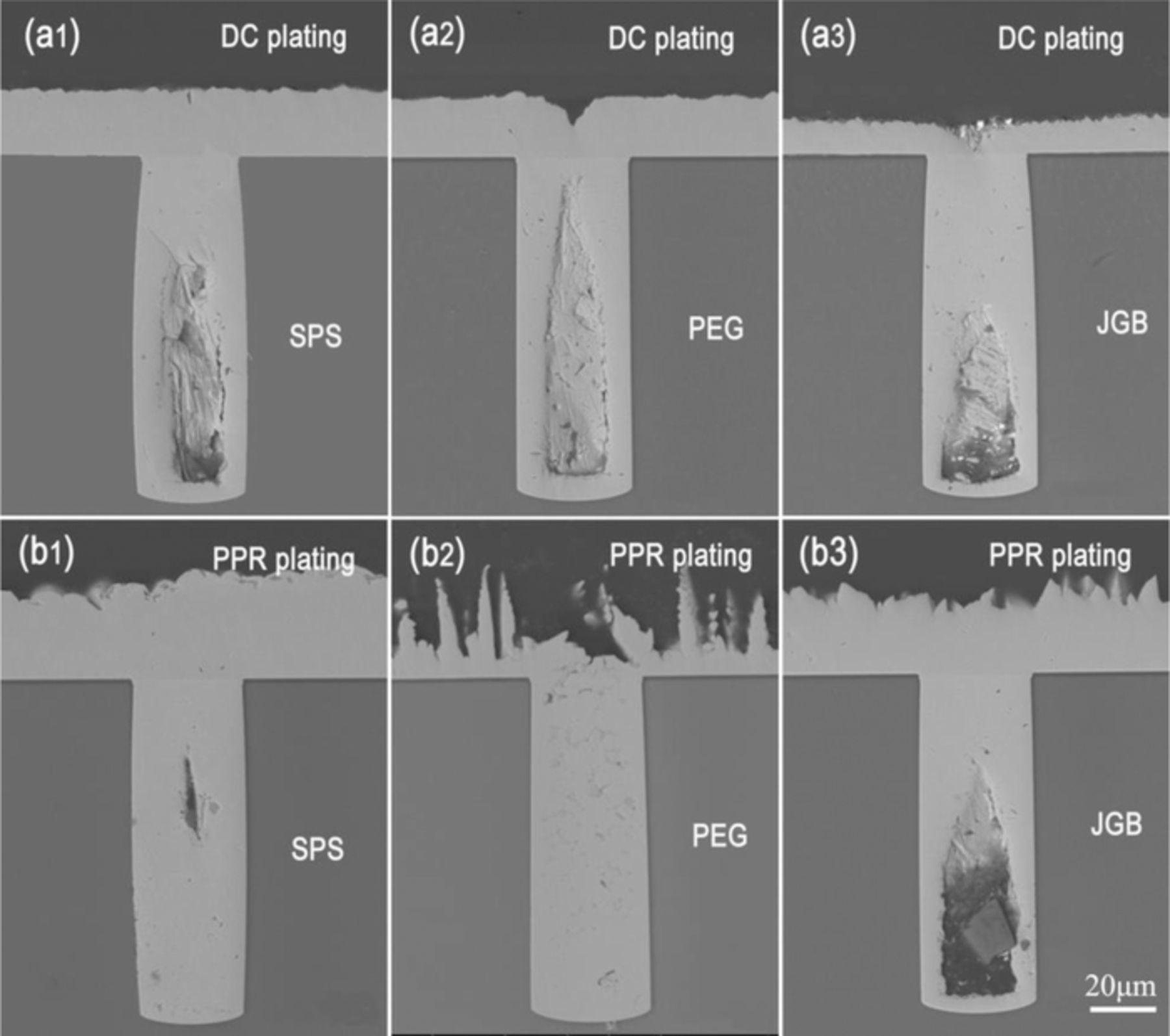
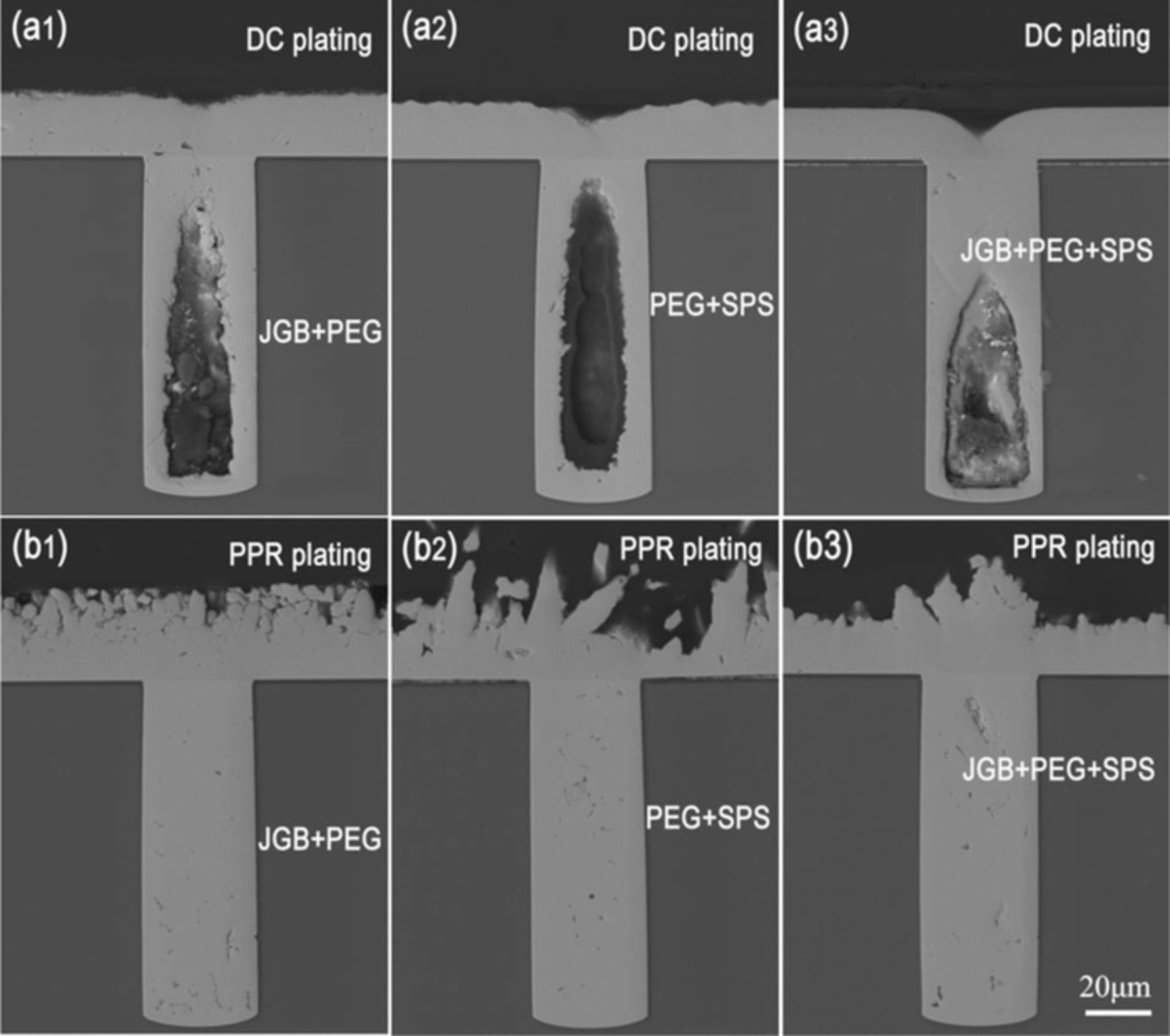
The TSV filling performances by DC and PPR electroplating using the Cu electrolytes containing single additives and 60 ppm Cl− were shown in Fig. 3. In DC electroplating, the voids appeared in filled profiles using any a single additive, as shown in Figs. 3a1–3a3. The occurrence of the voids was generally resulted from the failure of a bottom-up growth mode, i.e., the premature closure of microvia entrance.24 Figs. 3b1–3b3 showed the filling results by PPR electroplating. It was noted that the PPR electroplating could significantly improve the filling performance compared to those by DC plating in the electrolytes with single SPS or PEG additive. Especially using single PEG additive, a completely void-free filling effect could be successfully acquired in PPR plating. However, for the single JGB additive, the filling effect in PPR plating had no an obvious improvement compared to that in DC plating.
Figure 3. Cross-sectional observations of the filled TSV by DC and PPR plating using single additive: (a1) 10ppm SPS, DC plating, (a2) 200ppm PEG, DC plating, (a3) 10ppm JGB, DC plating, (b1) 10ppm SPS, PPR plating, (b2) 200ppm PEG, PPR plating, (b3) 10ppm JGB, PPR plating.
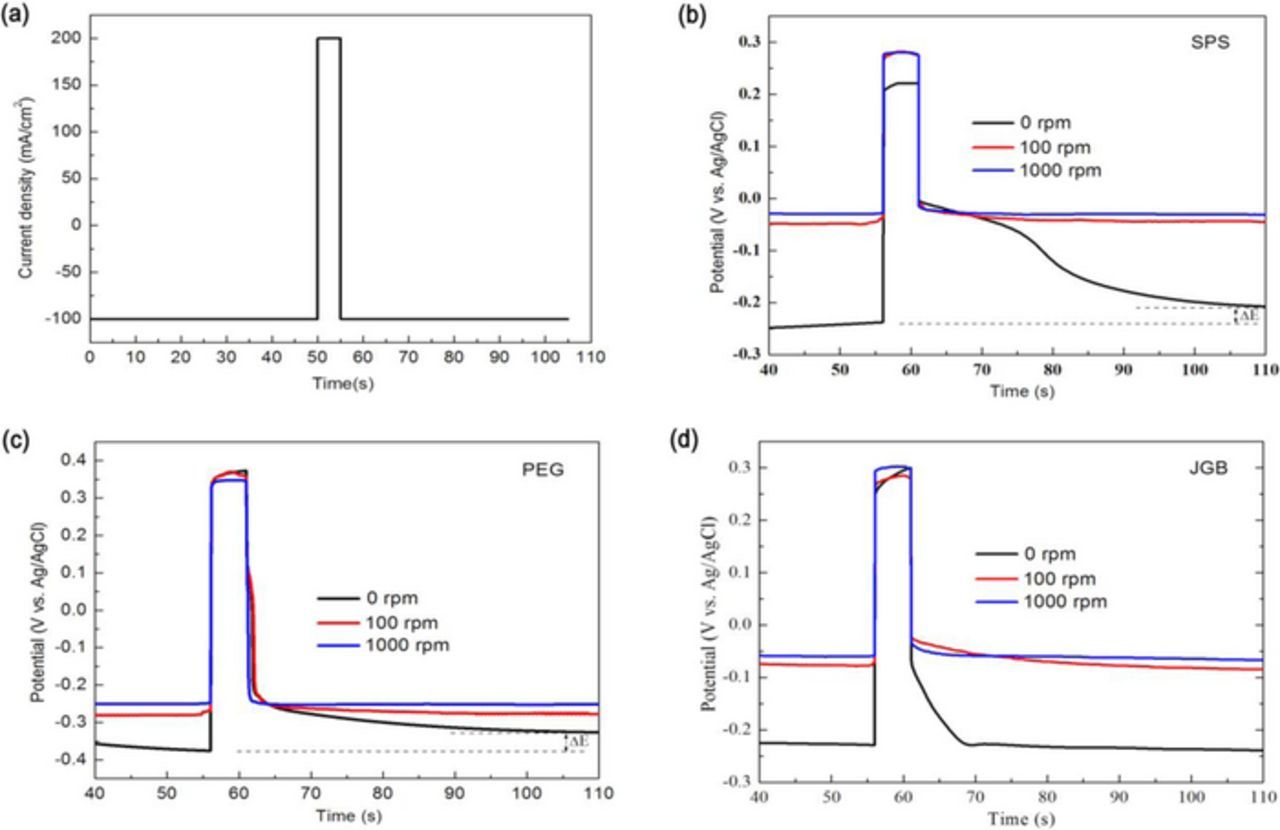
In chronopotentiometry measurements, the applied constant current profile was shown in Fig. 4a, where a short-time anodic current with a high current density was applied to simulate the reverse current in PPR plating. The effect of reverse current on the adsorption or desorption of additives was monitored through the potential variation. Figs. 4b and 4c showed the potential variation with time for the electrolytes with single additive SPS and PEG respectively. During the first deposition current for 50 s, the responding potential over working electrode reached an equilibrium value. Then after a reverse pulse, the potential shifted into a more positive value at the low rotation rates of 0 rpm or 100 rpm while almost kept constant at the high rotation rate of 1000 rpm. It meant that the deposition at the microvia bottom with a low convection force would be accelerated while the deposition rate near the microvia entrance with a high convection force kept unchanged. The accelerating effect at the bottom was generally helpful for a bottom-up filling mode, which well agreed with the improved filling performance by PPR plating compared to DC plating using single PEG or SPS additive. In contrast, for the electrolyte with single JGB additive, the potential hardly changed after reverse pulse at low rotation rates of 0 rpm or 100 rpm. It meant that the reverse pulse had no significant effect on the deposition rate at the microvia bottom, which accounted for a similar filling performance between PPR and DC plating using single JGB additive.
Figure 4. (a) The applied current in chronopotentiometry measurements, and the potential variation after anodic pulse current at varied rotation speeds of RDE in the electrolytes with single additive: (b) 10ppm SPS, (c) 200ppm PEG, (d) 10ppm JGB.
Chloride ion dependent adsorption mechanism of single PEG and SPS under reverse pulse
The mechanism of PEG adsorption on the cathode surface was dependent on the chloride ions.11,13,25–27 The PEG-Cu+ and PEG-Cu2+ complexes were the main existing forms of PEG in Cu electrolyte without Cl−, which absorbed on the cathode surface mainly by two acting forces, i.e., the Van der Waals force and the electrostatic force between the negatively charged cathode and positively charged PEG-Cu+ and PEG-Cu2+.13 In the electrolyte with Cl−, the adsorption of Cl− on the copper surface could immediately form Cu-Cl−,(i.e., CuCl precursor), which was demonstrated to be the adsorbing site for additives on the copper surface.11,25,26 Thus, the adsorption of PEG was mainly in the form of PEG-Cu+-Cl− which arose from the coordinating behavior of copper ions with PEG and Cl−. In this PEG-Cu+-Cl− complex, the Cl− played a role of bridge to connect the cathode and PEG.
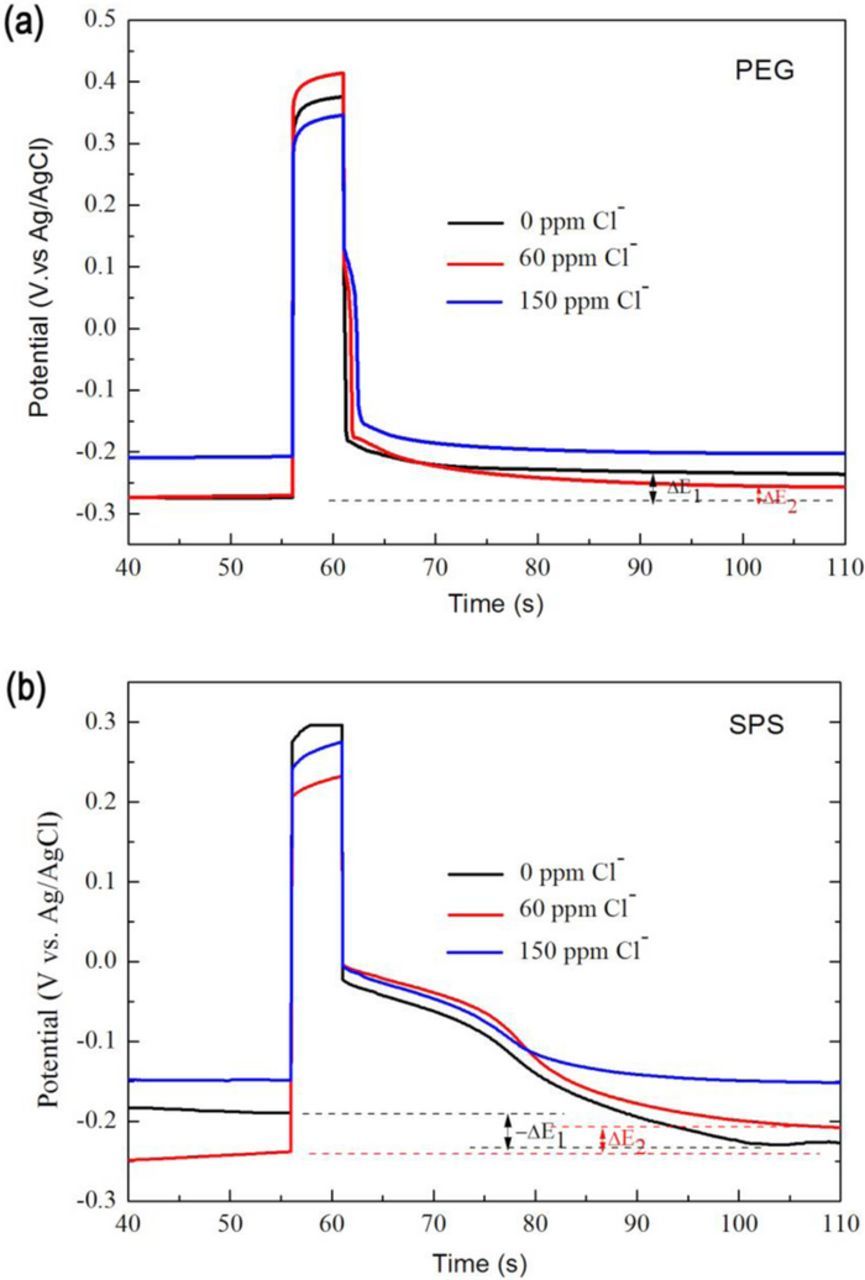
The influence of reverse pulse on the additive adsorption was related to the transient electrostatic force between the positively charged cathode and the complex. When the Cl− concentration was zero, the PEG-Cu+ was the only complex form. Due to the positive charge of the PEG-Cu+, the positive pulse current produced repulsive force to this complex, which result in the detachment of PEG from the cathode surface. Thus, with the reduction of this inhibitor, the potential exhibited a positive shift, as shown in Fig. 5a. In comparison, the positive pulse current produced an attractive force to the Cl− in the electrolyte. The attracted Cl− afforded more sites to form the PEG-Cu+-Cl− complex in the cathode, which was beneficial to the adsorption of PEG. When the Cl− concentration was 60 ppm, the complex was consisted of both PEG-Cu+ and PEG-Cu+-Cl−. If the amount of positively charged PEG-Cu+ was more than that of negatively charged PEG-Cu+-Cl−, the repelled PEG should be more than the attracted PEG by the positive pulse. Therefore, the adsorption of PEG was reduced in total, which could be reflected by the positive shift of potential, as shown in Fig. 5a. Due to carrying less positive charge, the amount of potential shift after reverse pulse at 60 ppm of Cl− was smaller than that at 0 ppm. If reducing the adsorption of this inhibitor at the microvia bottom, the filling performance could be improved, as shown in Figs. 3a2, 3b2. When the Cl− increased to a sufficient concentration of 150 ppm, the excessive adsorption sites resulted in a complete complex of PEG-Cu+-Cl− and also might reach a saturated adsorption. As a result, the positive pulse current would not exhibit an obvious effect on the PEG adsorption.
Figure 5. The potential variation after anodic pulse current in the electrolytes with varied Cl− concentrations and single additive: (a) 200ppm PEG, (b) 10ppm SPS. The chronoamperometry tests were carried out at 0rpm of RDE rotation speed.
The previous studies have shown that the adsorption of SPS was closely associated with the chloride ions.11,22,23,28,29 When the Cl− was added, a stable cuprous complex of CuCl would be formed on the surface, which was the preferential site for the adsorption of the SPS, forming a complex derivative of Cu-SPS-Cl−. The SPS could also directly adsorb on the sites of cuprous ions to form Cu+-SPS specie. Due to the transient electrostatic force, the positive pulse current will capture more Cl− to form Cu-SPS-Cl− complex while detach the positively charged Cu+-SPS complex. Therefore, the effect of positive pulse on the SPS adsorption was also determined by the Cl− concentration.
When the Cl− concentration was 60 ppm, it was thought that the amount of negatively charged Cu-SPS-Cl− was more than positively charged Cu+-SPS complex. Thus, the adsorption of SPS could be enhanced by the positive pulse, which could be reflected by the positive shift of potential, as shown in Fig. 5b. Due to the increased SPS adsorption at the bottom, the PPR electroplating could obtain a better void-free effect than DC electroplating, as shown in Figs. 3a1, 3b1. When the Cl− concentration was zero, the positive pulse current had an expelling effect to the positively charged Cu+-SPS complex, which led to a desorption effect of SPS. Thus the potential shifted into a more negative value after pulse current, as shown in Fig. 5b. When Cl− had an excessive concentration of 150 ppm, the Cu-SPS-Cl− complex could reach a saturated adsorption on the cathode, leaving little space for further adsorption by the electrostatic force. Therefore, the potential showed an imperceptible change after positive pulse current.
Besides the condition of excessive Cl− concentration, the adsorption of the Cu-SPS-Cl− or Cu-PEG-Cl− complexes could also reach a saturated adsorption under strong convection condition because the Cl− could be promptly provided for the formation of these complexes. Therefore, at high rotation speed of 1000 rpm, the potential exhibited an invisible change after the positive pulse current, as shown in Fig. 4. It appeared that the effect of reverse pulse on the adsorption of these complexes with Cl− was visible at the microvia bottom with weak convection force. Therefore, in PPR electroplating, it is important to optimize the Cl− concentration based on the corresponding PEG or SPS concentration, so that obtain an effect of promoting accelerator or reducing suppressor adsorption at the microvia bottom by reverse pulse, which is favor to a bottom-up filling mode.
Effect of reverse pulse on microvia filling performance using composite additives
Fig. 6 showed the filling results of TSV by DC and PPR electroplating in the Cu electrolytes containing two or three additives. There were large voids occurring within microvia in DC plating, as shown in Figs. 6a1–6a3, which demonstrated that the present additive formulas could not satisfy the requirement of bottom-up mode under the applied electroplating condition. In contrast, as shown in Figs. 6b1–6b3, the perfectly void-free filling results could be acquired by PPR plating using the same additives formulas.
Figure 6. Cross-sectional observations of the filled TSV by DC and PPR plating using composite additives: (a1) 200ppm PEG+10ppm JGB, DC plating, (a2) 200ppm PEG+10ppm SPS, DC plating, (a3) 200ppm PEG+10ppm SPS+10ppm JGB, DC plating, (b1) 200ppm PEG+10ppm JGB, PPR plating, (b2) 200ppm PEG+10ppm SPS, PPR plating, (b3) 200ppm PEG+10ppm SPS+10ppm JGB, PPR plating.
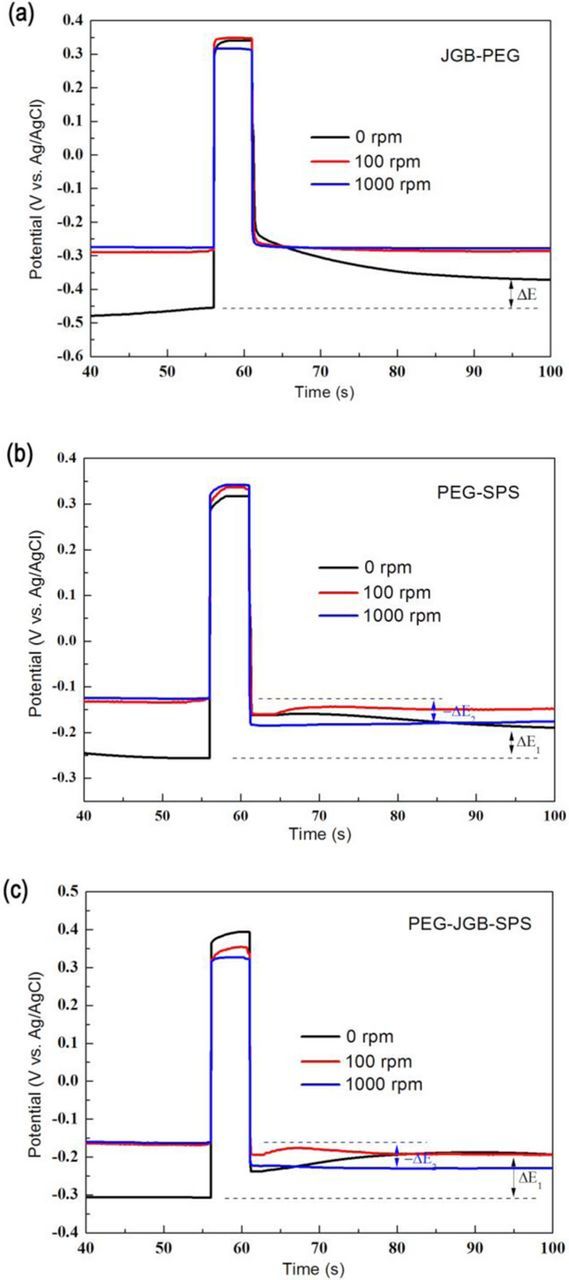
The chronopotentiometry curves of the PEG-JGB additive were shown in Fig. 7a. The potential became more positive after reverse pulse at low rotation speed and kept constant at high rotation speed. The more positive potential suggested that the inhibitor adsorption was reduced and consequently the deposition rate was improved at the microvia bottom, which was consistent with the greatly improved filling performance by PPR plating using PEG-JGB electrolyte. The effect of reverse pulse on the adsorption of PEG-SPS and PEG-JGB-SPS was revealed by the chronopotentiometry curves, as shown in Figs. 7b and 7c. After reverse pulse, the potential became more positive at low rotation rate. It was interestingly noted that the potential became more negative at high rotation speed, which was never found in any a single additive or the PEG-JGB at high rotation speed of 1000 rpm. This result meant that the Cu deposition was not only speeded up at the microvia bottom but also inhibited at the microvia entrance, which provided more favorable conditions for a void-free filling. In the following parts, we will discuss detailedly the operating mechanism of reverse pulse on the adsorption of composite additives within the microvia.
Figure 7. The potential variation after anodic pulse current at varied rotation speeds of RDE in the electrolytes with composite additives: (a) 200ppm PEG+10ppm JGB, (b) 200ppm PEG+10ppm SPS, (c) 200ppm PEG+10ppm JGB+10ppm SPS.
Adsorption behavior of composite additives of PEG-JGB under reverse pulse
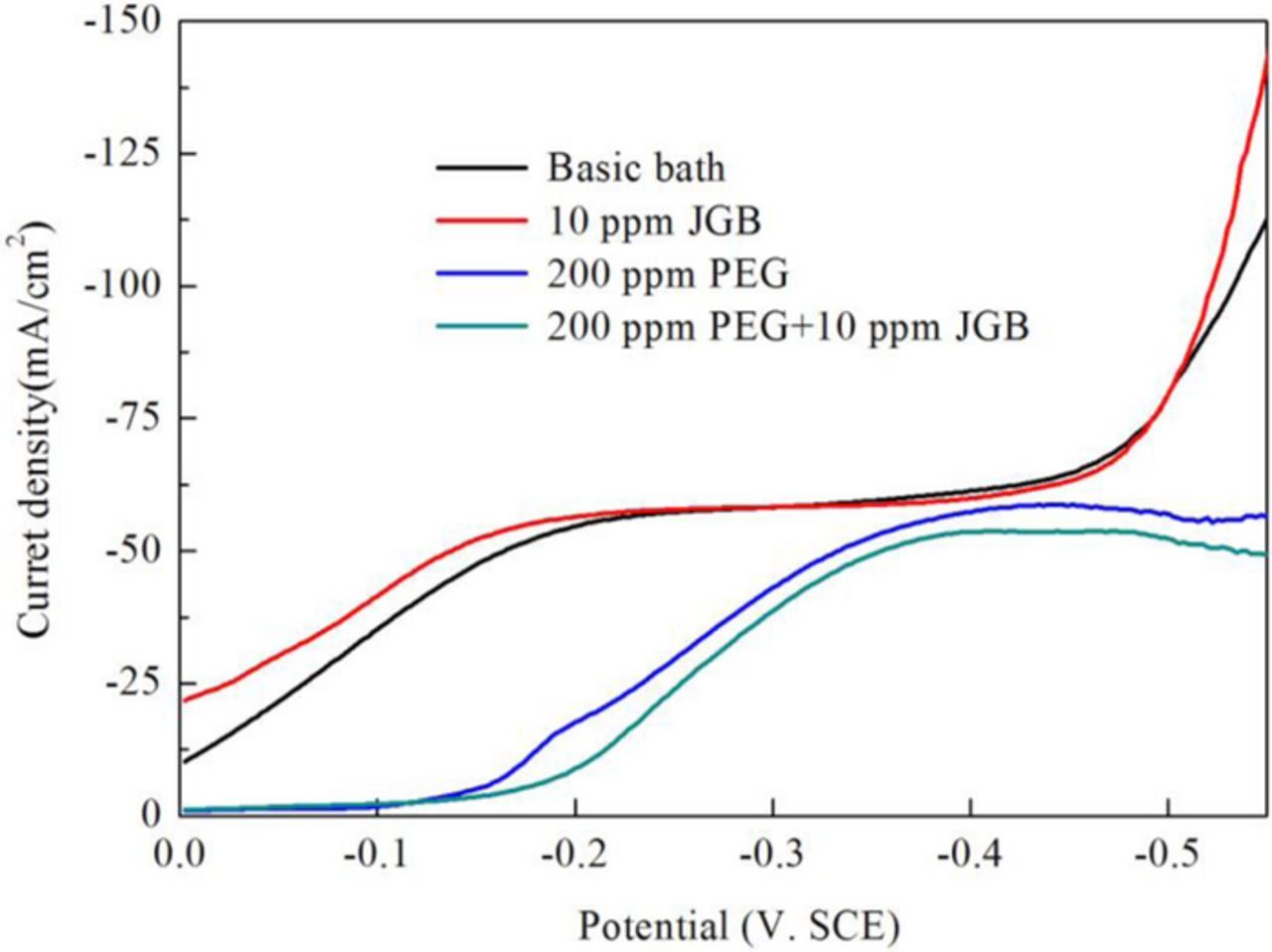
JGB belongs to the heterocyclic aromatic compounds containing quaternary ammonium, which can be adsorbed preferentially on the protrusion of cathode surface.30–32 Unlike the PEG or SPS, the JGB have not been found to form the complex with the Cu+ or Cl−. Therefore, the reverse pulse hardly had an effect on the adsorption behavior of single JGB, which had been testified by the unchanged microvia filling performance and the potential in chronopotentiometry curve after reverse pulse. As shown in Fig. 8, the single JGB did not exhibit a strong inhibition effect if the PEG was not present in the electrolyte. Compared to the single JGB, the PEG-JGB compositor exhibited a significant suppressing effect at the rotation speed of 100 rpm. Dow et al. thought that the inhibition effect was caused by the synergistic operation of JGB and PEG and this composite suppressor was found to be strongly convection-dependent.33 Like the PEG-Cu+-Cl−, the (JGB-PEG)-Cu+-Cl− complex was estimated to be positively charged when the Cl− concentration was 60 ppm. In the same way, it was thought that the positive pulse current would have an expelling effect on the (JGB-PEG)-Cu+-Cl− complex at the microvia bottom. On the other hand, due to the saturated adsorption at the entrance, the adsorption was hardly impacted by the positive pulse current. When the deposition was accelerated at the microvia bottom because of the detachment of this strong inhibitor of PEG-JGB, the bottom-up deposition was greatly promoted, which contributed to a perfectly void-free filling result by PPR electroplating using the JGB-PEG composite additive, as shown in Figs. 6a1, 6b1.
Figure 8. Linear sweep voltammograms of varied Cu electrolytes at 100 rpm of RDE rotation speed.
Competitive adsorption of SPS with PEG or PEG-JGB under reverse pulse
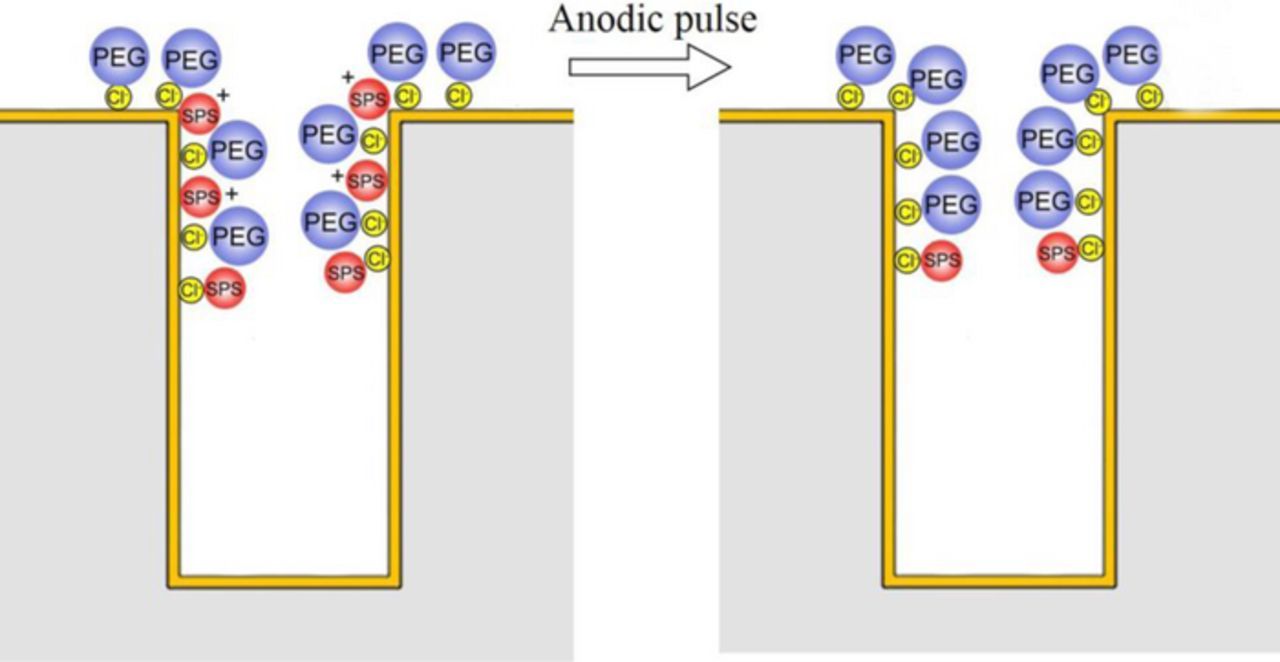
For the single additives or composite PEG-JGB, the reverse pulse had hardly influence on the potential at the high rotation of 1000 rpm because of enough supplement of Cl−. However, for the composite additive of PEG-SPS or PEG-JGB-SPS, the reverse pulse had a significant effect on the potential at the high rotation of 1000 rpm, as shown in Figs. 7b and 7c. It was known that the adsorption of PEG or PEG-JGB was strongly dependent on the convection force.33 Therefore, at the entrance with a strong convection force, the adsorption capability of PEG or PEG-JGB was much stronger than SPS. In the presence of PEG, this suppressor would preferentially form a dense layer of Cu+-PEG-Cl−. This layer could effectively block the transportation of Cl− to reach the copper surface and then few sites of Cl− were left for the SPS adsorption. The main existing form of SPS was the positively charged Cu+-SPS complex. Thus, the reverse pulse would easily detach this positively charged complex. The related operating mechanism was illustrated in Fig. 9. M. Hayase et al. also reported that the SPS could be desorbed at the microvia entrance by the reverse pulse in a PEG-JGB-SPS electrolyte,20,34 which well supported the present mechanism model. This model explained that the accelerator SPS could be detached at the entrance by the reverse pulse in the presence of sufficient Cl− and PEG or PEG-JGB. In the absence of PEG or PEG-JGB, the SPS could not be removed at the entrance by the reverse pulse even though sufficient Cl-, which had been affirmed by the above result, as shown in Fig. 4b. In total, the detachment of SPS at the microvia entrance by the reverse pulse was contributed to a weaker adsorption competitiveness of SPS against the PEG or PEG-JGB under strong convection force.
Figure 9. Schematic illustration of the detachment of SPS at microvia entrance by anodic pulse in the presence of PEG and sufficient chloride ion.
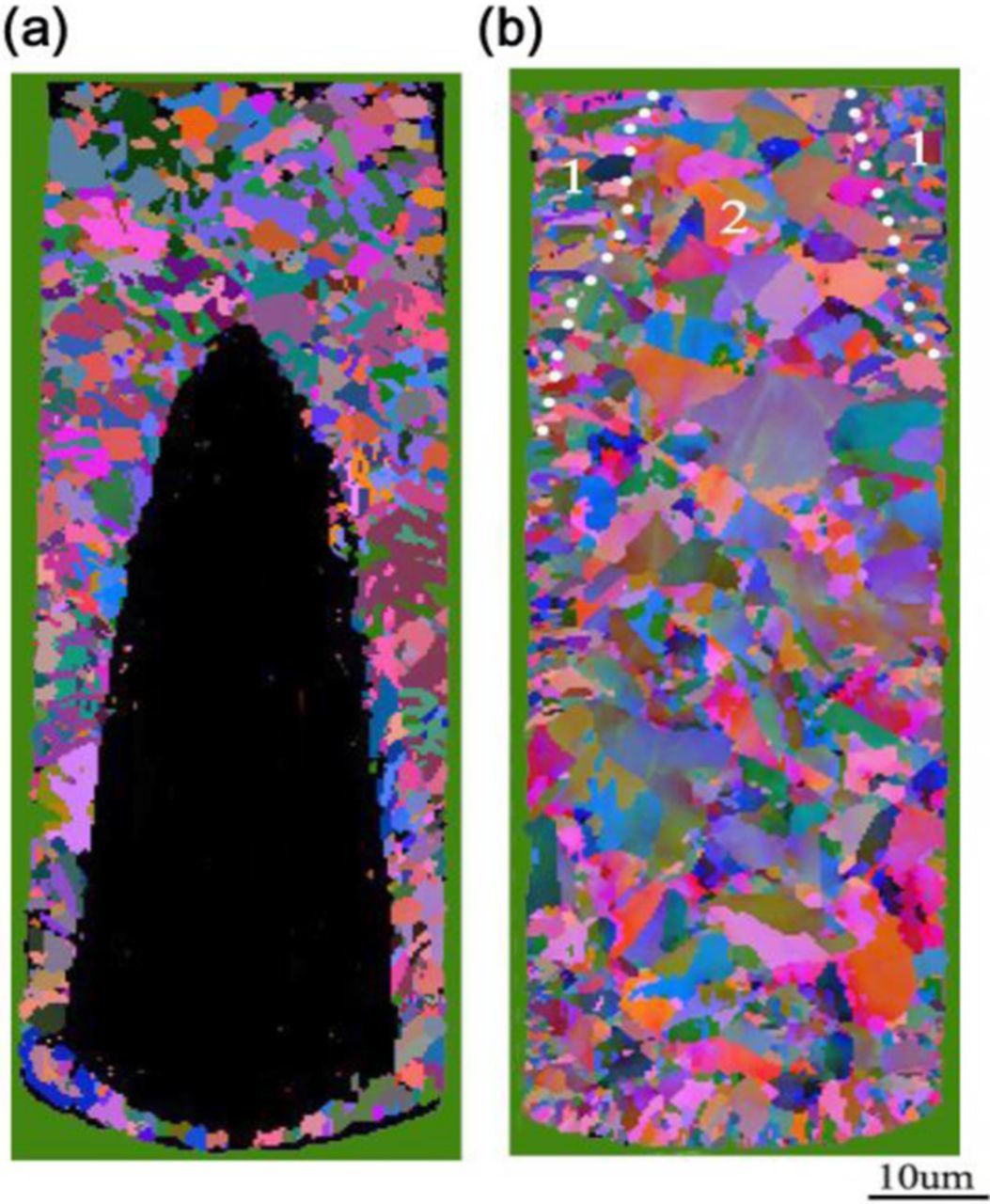
The filling performance and microstructures using DC and PPR plating in a PEG-JGB-SPS electrolyte were shown in Fig. 10. For DC electroplating, the occurrence of large void was resulted from the early closure at the microvia entrance. In the PPR plating, the detachment of SPS at the entrance and the enhanced adsorption of SPS at the bottom by reverse pulse greatly facilitated a void-free filling for the microvia. The grain size by DC plating had a relatively uniform distribution. However, in the PPR plating, the grain size in the edge zone was notably finer than that in the center zone, which reflected a selective adsorption behavior of additives in PPR plating. Compared to the grains in DC plating, the finer grain in zone 1 demonstrated that the Cu growth was dominated by the suppressor, which verified the fact of effective removing of the accelerator SPS by the reverse pulse at microvia entrance. The sustained acceleration on the upward-moving "V" shaped deposit surface speeded up grain growth.35,36 This rapid grain growth dominated by the accelerator could be reflected by the relatively coarse grain in the middle region (zone 2) along the central axis of "V" shaped filling profile, as shown in Fig. 10b.
Figure 10. EBSD grain maps of the filled Cu within TSV by electroplating using an electrolyte with composite additives of PEG-JGB-SPS: (a) DC plating, (b) PPR plating.
Conclusions
In this work, the operating mechanism of the reverse pulse on the additive adsorption within TSV were studied systematically. The effect of reverse pulse on the adsorption of the single PEG or SPS strongly depended on the Cl− concentration. When the Cl− was insufficient, the positively charged PEG-Cu+ or SPS-Cu+ complex was formed and the adsorption could be repelled by the reverse pulse. When the Cl− was sufficient, the negatively charged Cu-SPS-Cl− or Cu-PEG-Cl− was formed and the adsorption could be promoted. Based on that, in the case of 60 ppm Cl−, the reverse pulse could repel the PEG or promote adsorption of SPS at the microvia bottom, which was beneficial to a bottom-up filling mode. When the Cl− could be fully replenished at high speed rotation or excessive Cl− concentration, the effect of reverse pulse on the adsorption of single additives was not obvious.
The reverse pulse had no significant effect on the adsorption of single JGB. In comparison, the composite JGB-PEG inhibitor could be repelled by the reverse pulse at the microvia bottom when Cl− concentration was 60 ppm, which was helpful for the void-free filling. A dense layer of Cu+-Cl−-PEG preferentially adsorbed and then effectively blocked the transportation of Cl− to the copper surface. Few sites of Cl− were left for the SPS adsorption and the SPS adsorbed mainly in the form of Cu+-SPS. The reverse pulse could detach the positively charged Cu+-SPS at the microvia entrance, which greatly improved the performance of void free filling. This mechanism based on competitive adsorption explained that only in the presence of PEG the SPS could be effectively removed by reverse pulse at the microvia entrance.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (NSFC), under the grant No. 51471180, and Science and Technology Program of Shenyang, under the grant No. F16-205-1-18.
ORCID
Q. S. Zhu 0000-0003-1379-7365