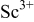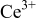Abstract
In this article, we discuss the crystal chemistry and luminescence properties of  activated silicate garnets. Our results indicate that the two previously reported end members of this family,
activated silicate garnets. Our results indicate that the two previously reported end members of this family,  and
and  , form a complete solid solution, and
, form a complete solid solution, and  primarily substitutes for
primarily substitutes for  in the octahedral sites with charge compensation by
in the octahedral sites with charge compensation by  substitution in the dodecahedral sites. Within this solid solution, there is a continuous shift in the lowest energy
substitution in the dodecahedral sites. Within this solid solution, there is a continuous shift in the lowest energy 
 excitation band and the
excitation band and the 
 emission band toward higher energy as the composition is varied between
emission band toward higher energy as the composition is varied between  and
and  .
.
Export citation and abstract BibTeX RIS
Solid-state lighting based upon a combination of violet/blue light-emitting diodes (LEDs) and downconversion phosphors has created enormous commercial interest because of potentially higher efficacies and long lifetimes for these light sources. Lamps based upon phosphor downconversion of blue LEDs generally use  garnet
garnet  phosphors. The lowest allowed
phosphors. The lowest allowed 
 transition strongly absorbs blue radiation and efficiently downconverts this radiation into yellow light through the reverse
transition strongly absorbs blue radiation and efficiently downconverts this radiation into yellow light through the reverse  emission transition.1 It is possible to design phosphors that have emission colors that are redshifted vs
emission transition.1 It is possible to design phosphors that have emission colors that are redshifted vs  by understanding the relationship between crystal chemistry and luminescence in these phosphors.2 The garnet structure can be represented by the general formula
by understanding the relationship between crystal chemistry and luminescence in these phosphors.2 The garnet structure can be represented by the general formula  , where {A} cations are eight-coordinated in dodecahedra, [B] cations are octahedrally coordinated, and (C) cations are tetrahedrally coordinated. The position of the
, where {A} cations are eight-coordinated in dodecahedra, [B] cations are octahedrally coordinated, and (C) cations are tetrahedrally coordinated. The position of the  5d levels is strongly influenced by the host lattice selection,3–5 and hence, the
5d levels is strongly influenced by the host lattice selection,3–5 and hence, the  emission could be controlled through careful substitutions in the dodecahedral, octahedral, and tetrahedral sites. In previous work, we showed that it is possible to synthesize
emission could be controlled through careful substitutions in the dodecahedral, octahedral, and tetrahedral sites. In previous work, we showed that it is possible to synthesize  garnets, specifically
garnets, specifically  , that have redshifted emission
, that have redshifted emission  vs typical
vs typical  phosphors; the lower energy for
phosphors; the lower energy for  emission was attributed to the larger crystal field splitting of the two lowest energy 5d levels.6
emission was attributed to the larger crystal field splitting of the two lowest energy 5d levels.6  replacement of
replacement of  led to a blueshift in the
led to a blueshift in the  emission spectra, but with a lower room and high temperature (HT) efficiency. Other articles on
emission spectra, but with a lower room and high temperature (HT) efficiency. Other articles on  luminescence in silicate garnets, such as
luminescence in silicate garnets, such as  , have a higher energy
, have a higher energy 
 emission band
emission band  with excellent HT efficiencies.7, 8 The differences in the position of the
with excellent HT efficiencies.7, 8 The differences in the position of the 
 emission band between these pure silicate garnets lead to the question of how well
emission band between these pure silicate garnets lead to the question of how well  emission can be tuned in these garnets vs the aluminate garnets, and if there are any apparent trade-offs in the efficiency of these materials, especially at HTs. We address these questions in this article by evaluating nominal compositions within the
emission can be tuned in these garnets vs the aluminate garnets, and if there are any apparent trade-offs in the efficiency of these materials, especially at HTs. We address these questions in this article by evaluating nominal compositions within the  system to determine the range of solid solution between
system to determine the range of solid solution between  and
and  and to understand the effects of composition on
and to understand the effects of composition on  emission, excitation, and efficiency. Our results suggest that there is a full solid solution between the
emission, excitation, and efficiency. Our results suggest that there is a full solid solution between the  and
and  silicate garnets with limited
silicate garnets with limited  solubility on the dodecahedral {A} sites. Within these compositions, the emission maximum for
solubility on the dodecahedral {A} sites. Within these compositions, the emission maximum for 
 emission can be fully tuned within the range of 505–605 nm with corresponding shifts in the lowest energy
emission can be fully tuned within the range of 505–605 nm with corresponding shifts in the lowest energy 
 excitation band. However, unlike
excitation band. However, unlike  additions to
additions to  ,6 the blueshift in
,6 the blueshift in  emission in
emission in  compositions does not come with any losses in phosphor efficiency at room or elevated temperatures. Potential reasons for
compositions does not come with any losses in phosphor efficiency at room or elevated temperatures. Potential reasons for  luminescence quenching and the changes in the position of the lowest energy
luminescence quenching and the changes in the position of the lowest energy  5d level are also discussed.
5d level are also discussed.
Experimental
Powder samples with nominal compositions of  and
and  were synthesized by conventional solid-state reactions starting from high purity
were synthesized by conventional solid-state reactions starting from high purity  (PIDC),
(PIDC),  (Aldrich), MgO (Aldrich),
(Aldrich), MgO (Aldrich),  (PIDC),
(PIDC),  (Aldrich), and fumed
(Aldrich), and fumed  (Cabosil). The reactants were mixed well and heated in
(Cabosil). The reactants were mixed well and heated in  mixtures at
mixtures at  for 5–10 h. The compositions discussed in this article are referred to by their nominal starting compositions. Powder X-ray diffraction (XRD) patterns were recorded for
for 5–10 h. The compositions discussed in this article are referred to by their nominal starting compositions. Powder X-ray diffraction (XRD) patterns were recorded for  using a Philips X'pert diffractometer (
using a Philips X'pert diffractometer ( radiation). Lattice parameters were obtained from powder matching of the XRD data using the Fullprof program. The emission and excitation spectra were measured on pressed phosphor powders in an aluminum plaque using an F4500 Hitachi fluorescence spectrophotometer. All measurements were made at room temperature unless otherwise mentioned. The relative quantum efficiency (QE) was measured with respect to a commercial
radiation). Lattice parameters were obtained from powder matching of the XRD data using the Fullprof program. The emission and excitation spectra were measured on pressed phosphor powders in an aluminum plaque using an F4500 Hitachi fluorescence spectrophotometer. All measurements were made at room temperature unless otherwise mentioned. The relative quantum efficiency (QE) was measured with respect to a commercial  phosphor with
phosphor with  (Kodak) as a reflection standard. HT luminescence measurements were made on pressed powders in an aluminum plaque connected with resistive heaters and a thermocouple that were attached to a standard Watlow temperature controller.
(Kodak) as a reflection standard. HT luminescence measurements were made on pressed powders in an aluminum plaque connected with resistive heaters and a thermocouple that were attached to a standard Watlow temperature controller.
Results and Discussion
Phase characterization and  site occupation
site occupation
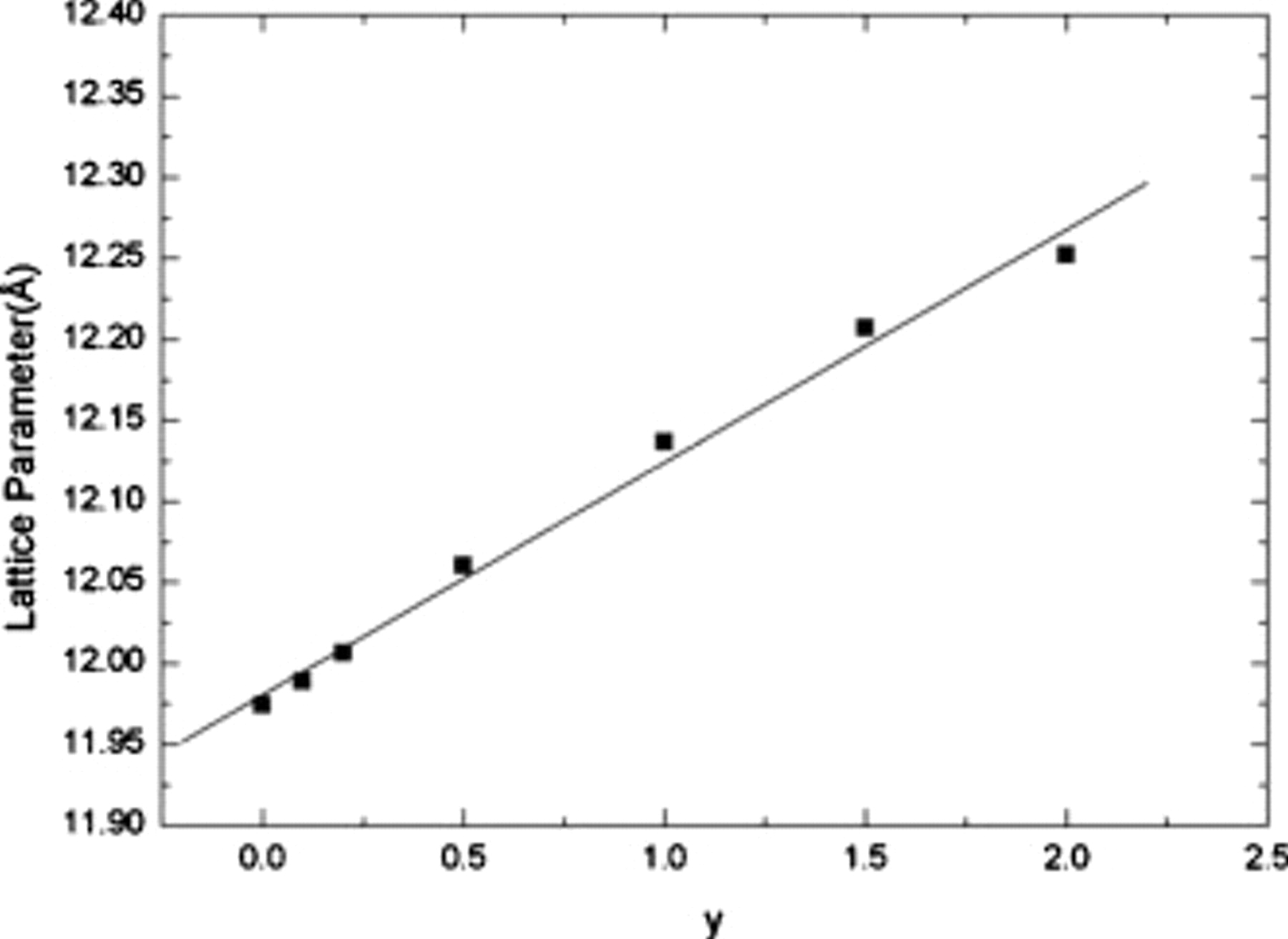
Silicate garnet synthesis under ambient pressure conditions6–10 is more challenging vs aluminate and germanate garnets with limits on the compositional space. However, we have demonstrated that silicate garnet synthesis at ambient pressure tends to favor compositions with smaller  ions such as
ions such as  , partially occupying the {A} (dodecahedral) sites.6 In addition, larger ions at the [B] octahedral sites also tend to stabilize garnet phase formation.7–10 This interplay between dopant ions and their occupancy in the garnet plays a role in the stability and phase purity of the garnets; it is therefore of interest to determine which site
, partially occupying the {A} (dodecahedral) sites.6 In addition, larger ions at the [B] octahedral sites also tend to stabilize garnet phase formation.7–10 This interplay between dopant ions and their occupancy in the garnet plays a role in the stability and phase purity of the garnets; it is therefore of interest to determine which site  primarily occupies in the
primarily occupies in the  garnet. Typically,
garnet. Typically,  substitutes into the [B] octahedral site in garnets,10 but hydrothermally synthesized garnet compositions such as
substitutes into the [B] octahedral site in garnets,10 but hydrothermally synthesized garnet compositions such as  11 show that
11 show that  can occupy the dodecahedral site for certain compositions and synthesis conditions. Therefore, compositions were made where
can occupy the dodecahedral site for certain compositions and synthesis conditions. Therefore, compositions were made where  is nominally substituted for
is nominally substituted for  on the dodecahedral site (i.e.,
on the dodecahedral site (i.e.,  ) and also where
) and also where  is nominally substituted for
is nominally substituted for  on the octahedral site with charge compensation through the
on the octahedral site with charge compensation through the  and
and  levels (i.e.,
levels (i.e.,  ). Figure 1 shows the comparison of the XRD patterns of (a)
). Figure 1 shows the comparison of the XRD patterns of (a)  , (b)
, (b)  , and (c)
, and (c)  . The primary phase in all of these samples are garnets with a small amount of secondary apatite phase present as previously reported.6 In
. The primary phase in all of these samples are garnets with a small amount of secondary apatite phase present as previously reported.6 In  samples, apatite is the only secondary phase for
samples, apatite is the only secondary phase for  . However, an additional
. However, an additional  impurity phase is also observed for
impurity phase is also observed for  in these samples. This indicates a limited solid solubility of
in these samples. This indicates a limited solid solubility of  in dodecahedral sites. Further, the garnet lattice parameter increases in all compositions where
in dodecahedral sites. Further, the garnet lattice parameter increases in all compositions where  nominally replaces
nominally replaces  (Table I). This does not correlate to the substitution of
(Table I). This does not correlate to the substitution of  by
by  , because the
, because the  (
( 12) ionic radius is significantly smaller compared to that of
12) ionic radius is significantly smaller compared to that of  (
( 12). However, an increase in lattice parameters is expected, if
12). However, an increase in lattice parameters is expected, if  (
( 12) replaces
12) replaces  (
( 12) on the octahedral site and
12) on the octahedral site and  (
( 12) replaces
12) replaces  for charge compensation. In principle, this can lead to additional impurity phases beyond apatite whenever
for charge compensation. In principle, this can lead to additional impurity phases beyond apatite whenever  nominally replaces
nominally replaces  , in contradiction to our observations of only an apatite secondary phase for
, in contradiction to our observations of only an apatite secondary phase for  . However, apatites can have significant nonstoichiometry and compositional flexibility,13 and we surmise that the additional
. However, apatites can have significant nonstoichiometry and compositional flexibility,13 and we surmise that the additional  that is not incorporated into the garnet phase is incorporated into the apatite at lower levels of
that is not incorporated into the garnet phase is incorporated into the apatite at lower levels of  substitution.
substitution.
Figure 1. Powder XRD pattern of (a)  , (b)
, (b)  , and (c)
, and (c)  . The symbol ∗ represents peaks due to a secondary apatite phase.
. The symbol ∗ represents peaks due to a secondary apatite phase.
Table I. Lattice parameters of  .
.
 | Lattice parameter (Å) |
|---|---|
| 0 | 11.9743(2) |
| 0.1 | 11.9884(3) |
| 0.2 | 11.9947(3) |
| 0.5 | 12.0062(3) |
| 1 | 12.0706(3) |
Unlike nominal compositions where  replaces
replaces  in
in  , the XRD patterns indicate that
, the XRD patterns indicate that  substitution in the octahedral sites (nominal compositions of
substitution in the octahedral sites (nominal compositions of  ) does not increase the relative intensity of additional impurity or secondary phase peaks for
) does not increase the relative intensity of additional impurity or secondary phase peaks for  . The expansion in lattice parameter with
. The expansion in lattice parameter with  substitution for
substitution for  in
in  is clearly seen in the
is clearly seen in the  compositions throughout the entire range of compositions from
compositions throughout the entire range of compositions from 
 to
to 
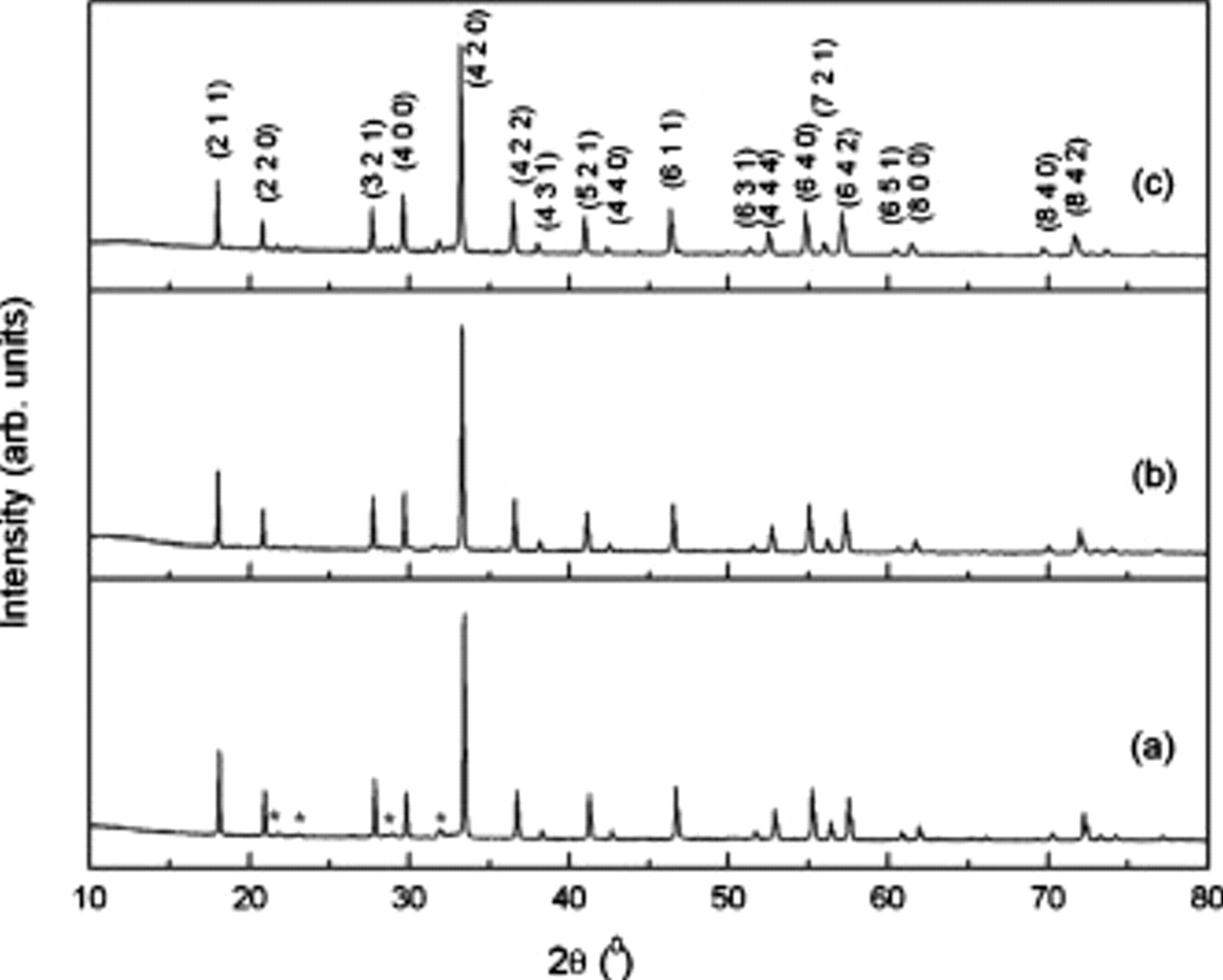
 (Table II) with the lattice parameters for the end-member compositions in reasonable agreement with previous results.6, 7 The lattice parameter vs the nominal
(Table II) with the lattice parameters for the end-member compositions in reasonable agreement with previous results.6, 7 The lattice parameter vs the nominal  substitution follows Vegard's law (Fig. 2), confirming the complete solid solubility between
substitution follows Vegard's law (Fig. 2), confirming the complete solid solubility between  and
and  .
.
Table II. Lattice parameters of  .
.
 | Lattice parameter (Å) |
|---|---|
| 0 | 11.9743(2) |
| 0.1 | 11.9890(2) |
| 0.2 | 12.0066(2) |
| 0.5 | 12.0606(2) |
| 1 | 12.1371(3) |
| 1.5 | 12.2070(3) |
| 2 | 12.2521(2) |
Figure 2. Variation in lattice parameter as a function of composition for the samples  .
.
 luminescence in these silicate garnets
luminescence in these silicate garnets
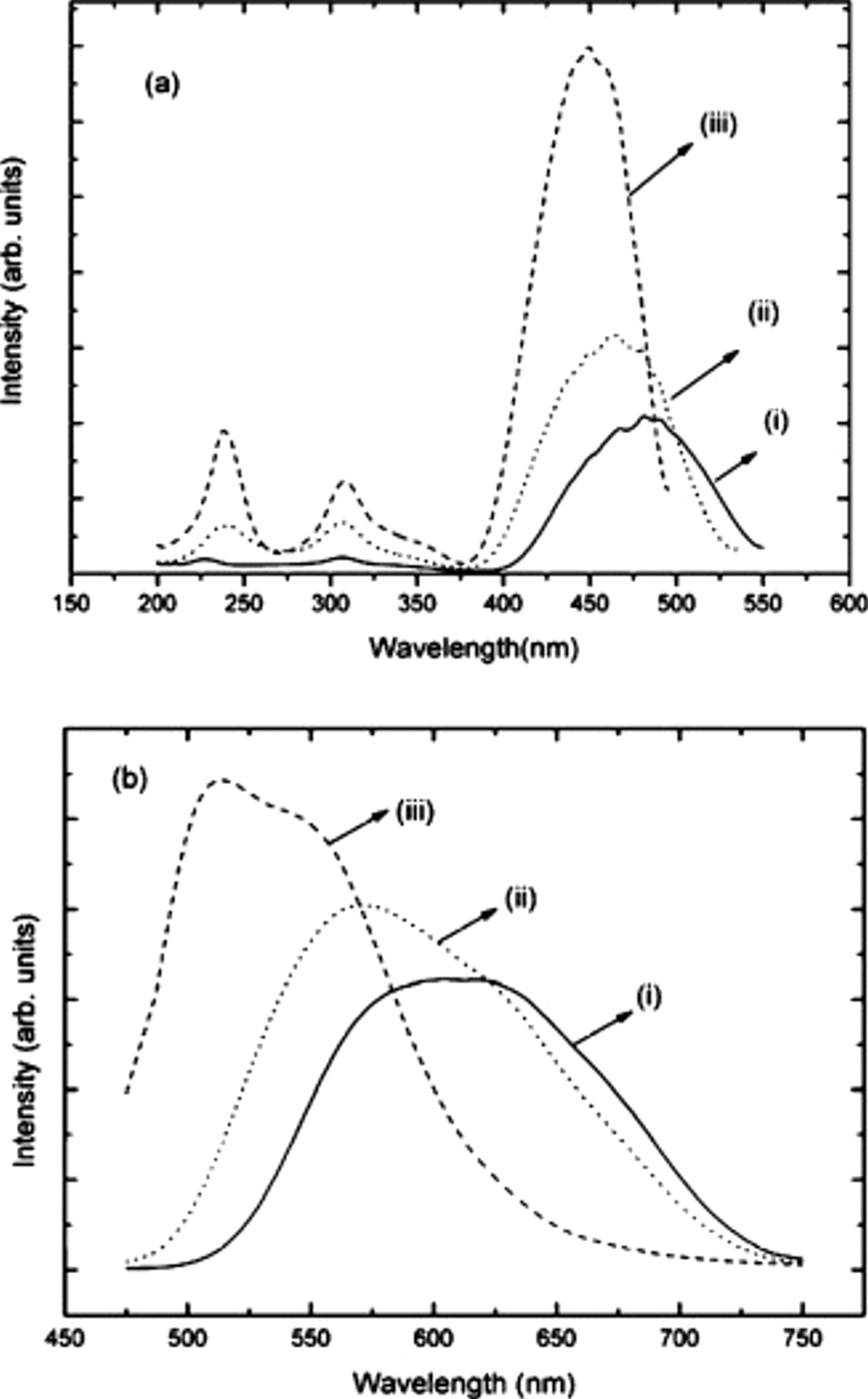
The body color of  is yellow-orange, due to the lowest energy
is yellow-orange, due to the lowest energy 
 absorption in the blue spectral region, with a broadband emission spectrum
absorption in the blue spectral region, with a broadband emission spectrum  and an emission maximum of
and an emission maximum of  .6 For
.6 For  compositions, the lowest energy
compositions, the lowest energy 
 absorption is at higher energies with larger values of
absorption is at higher energies with larger values of  , giving a more yellow-green body color (Fig. 3a). Correspondingly, the emission spectra are at higher energies with higher
, giving a more yellow-green body color (Fig. 3a). Correspondingly, the emission spectra are at higher energies with higher  content in
content in  (Fig. 3b). The
(Fig. 3b). The  emission color can be tuned from orange to blue-green emission by systematically replacing
emission color can be tuned from orange to blue-green emission by systematically replacing  for
for  and
and  for
for  in
in  . For all compositions, the emission spectrum can be deconvoluted into two Gaussians separated by
. For all compositions, the emission spectrum can be deconvoluted into two Gaussians separated by  . This is typical for the
. This is typical for the 
 emission and is indicative of emission from the lowest energy 5d level to the
emission and is indicative of emission from the lowest energy 5d level to the  and
and  levels. The tunable emission characteristics of these silicate garnet enable flexibility in designing potential LED lamps when using either single phosphors or blends of multiple phosphors. There is no apparent change in the Stokes shift with composition, although an accurate determination of the Stokes shift in these materials is difficult to assess due to severe inhomogeneous broadening that comes from the disorder on the dodecahedral and octahedral sites in these garnets.6–8
levels. The tunable emission characteristics of these silicate garnet enable flexibility in designing potential LED lamps when using either single phosphors or blends of multiple phosphors. There is no apparent change in the Stokes shift with composition, although an accurate determination of the Stokes shift in these materials is difficult to assess due to severe inhomogeneous broadening that comes from the disorder on the dodecahedral and octahedral sites in these garnets.6–8
Figure 3. (a) Excitation  and (b) emission
and (b) emission  spectra of
spectra of  with increasing Sc content, where (i)
with increasing Sc content, where (i)  , (ii)
, (ii)  , and (iii)
, and (iii)  . The sharp lines in the excitation spectra are experimental artifacts from the Xe lamp source.
. The sharp lines in the excitation spectra are experimental artifacts from the Xe lamp source.
We first assess the reasons for the higher energy  emission and absorption with a higher
emission and absorption with a higher  in the
in the  compositions. The energy of the lowest 5d level for
compositions. The energy of the lowest 5d level for  in the inorganic hosts is mainly dependent upon two factors: the 5d centroid shift from the
in the inorganic hosts is mainly dependent upon two factors: the 5d centroid shift from the  -free ion level and the crystal field splitting of the 5d levels.3–5 We start by analyzing the
-free ion level and the crystal field splitting of the 5d levels.3–5 We start by analyzing the  5d centroid shift in the end members of this system,
5d centroid shift in the end members of this system,  and
and  . The
. The  centroid shift in
centroid shift in  was estimated at
was estimated at  ,
,  smaller than the estimates for typical
smaller than the estimates for typical  garnets.6 Similar calculations using the crystallographic data for
garnets.6 Similar calculations using the crystallographic data for  9 give a centroid shift of
9 give a centroid shift of  , primarily from a longer
, primarily from a longer  bond length. This difference in the centroid shift therefore accounts for at least part of the energy difference between the lowest energy
bond length. This difference in the centroid shift therefore accounts for at least part of the energy difference between the lowest energy 
 transition in
transition in  and
and  . Typically, the
. Typically, the  crystal field splitting also has an inverse relationship with the
crystal field splitting also has an inverse relationship with the  –ligand bond length,3–5 which would imply a smaller crystal field splitting as the composition moves toward
–ligand bond length,3–5 which would imply a smaller crystal field splitting as the composition moves toward  . This is apparently the case in these garnets because the splitting between the lowest two 5d levels in
. This is apparently the case in these garnets because the splitting between the lowest two 5d levels in  is
is  and becomes gradually larger for lower
and becomes gradually larger for lower  values in
values in  (Fig. 3a). Therefore, it is likely that the blueshift of the
(Fig. 3a). Therefore, it is likely that the blueshift of the  emission and excitation spectra when substituting
emission and excitation spectra when substituting  into
into  is due to a combination of a smaller
is due to a combination of a smaller  5d centroid shift and crystal field splitting from the longer
5d centroid shift and crystal field splitting from the longer  bond length. The relationship between crystal field splitting and
bond length. The relationship between crystal field splitting and  –ligand bond length in these silicate garnets is typically observed for
–ligand bond length in these silicate garnets is typically observed for  luminescence,3–5 but differs from the
luminescence,3–5 but differs from the  garnets where larger {A} cations redshift the
garnets where larger {A} cations redshift the  emission and excitation spectra.2, 14, 15
emission and excitation spectra.2, 14, 15
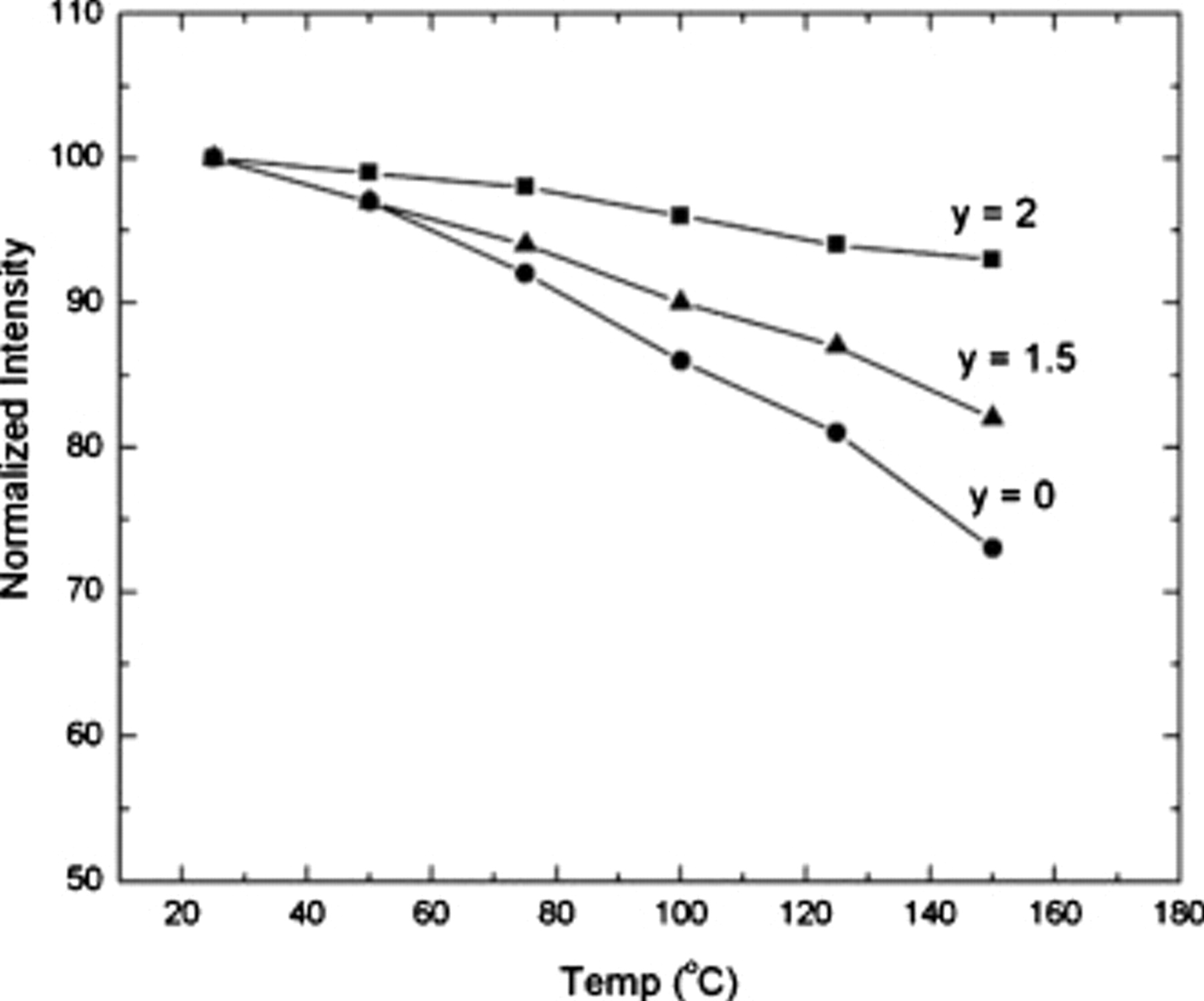
We finally discuss the QE of these phosphors at room and elevated temperatures. The QE increases with Sc substitution from 70 to 80% of  . This is a promising value considering that the phosphor synthesis is not optimized for efficiency. It is also important to account for quenching at HTs due to the heat generated by the high power violet/blue LEDs and from the phosphor downconversion. Though parallel efforts are being made to address thermal management issues in LED packages,16 it is essential to have phosphors retain their efficiency at elevated temperatures to address some of these issues.
. This is a promising value considering that the phosphor synthesis is not optimized for efficiency. It is also important to account for quenching at HTs due to the heat generated by the high power violet/blue LEDs and from the phosphor downconversion. Though parallel efforts are being made to address thermal management issues in LED packages,16 it is essential to have phosphors retain their efficiency at elevated temperatures to address some of these issues.  has reduced HT quenching compared to
has reduced HT quenching compared to  6–8 and this trend is also reflected within the solid solution compositions (Fig. 3). Hence, increasing
6–8 and this trend is also reflected within the solid solution compositions (Fig. 3). Hence, increasing  and
and  concentrations in
concentrations in  not only shifts the emission peak position to higher energy, it also improves phosphor efficiency at elevated temperatures (see Fig. 4). The relationship between absorption/emission position and HT efficiency potentially indicates that
not only shifts the emission peak position to higher energy, it also improves phosphor efficiency at elevated temperatures (see Fig. 4). The relationship between absorption/emission position and HT efficiency potentially indicates that  luminescence quenching occurs by a level crossing mechanism as proposed in the aluminate garnets15, 17 and garnets doped with
luminescence quenching occurs by a level crossing mechanism as proposed in the aluminate garnets15, 17 and garnets doped with  .18 As noted above, the synthesis of these phosphors has not been completely optimized, so further improvements in QE at room temperature and HT could be possible.
.18 As noted above, the synthesis of these phosphors has not been completely optimized, so further improvements in QE at room temperature and HT could be possible.
Figure 4. Integrated luminescence intensity  vs temperature for
vs temperature for  , where
, where  , 1.5, and 2.
, 1.5, and 2.
Conclusions
In this article, we have investigated the crystal chemistry and  luminescence properties within silicate garnets that can be synthesized at ambient pressure. There is strong evidence toward a continuous solid solution between the previously reported
luminescence properties within silicate garnets that can be synthesized at ambient pressure. There is strong evidence toward a continuous solid solution between the previously reported  5 and
5 and  .6, 7 Correspondingly,
.6, 7 Correspondingly,  substitution in these silicate garnets has a strong preference toward the octahedral site. We have also demonstrated that as the composition moves through this solid solution, the
substitution in these silicate garnets has a strong preference toward the octahedral site. We have also demonstrated that as the composition moves through this solid solution, the  emission continuously shifts, allowing for color flexibility in different LED lamp applications and that the HT efficiency of these materials also improves with higher
emission continuously shifts, allowing for color flexibility in different LED lamp applications and that the HT efficiency of these materials also improves with higher  levels.
levels.
Acknowledgments
The authors thank E. A. Bachniak for the initial sample synthesis and Digamber Porob and Emil Radkov for useful discussions. This work was supported by GE Lumination.
GE Global Research assisted in meeting the publication costs of this article.