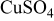Abstract
Scanning electrochemical microscopy (SECM), in the substrate generation–tip collection (SG-TC) mode, has been used to detect the cuprous ion  intermediate formed during the course of electrodeposition of Cu metal from aqueous
intermediate formed during the course of electrodeposition of Cu metal from aqueous  solution. Addition of chloride is confirmed to strongly stabilize the
solution. Addition of chloride is confirmed to strongly stabilize the  ion in aqueous solution and enhance the rate of Cu electrodeposition. This SECM method in the SG-TC mode offers an alternative to the rotating ring disk electrode (RRDE) technique for in situ studies on the effect of plating bath additives in metal electrodeposition. An attractive feature of the SECM relative to the RRDE method is that it allows qualitative aspects of the electrodeposition process to be studied in close proximity to the substrate in a simple and direct fashion using an inexpensive probe, and without the need for forced convection.
ion in aqueous solution and enhance the rate of Cu electrodeposition. This SECM method in the SG-TC mode offers an alternative to the rotating ring disk electrode (RRDE) technique for in situ studies on the effect of plating bath additives in metal electrodeposition. An attractive feature of the SECM relative to the RRDE method is that it allows qualitative aspects of the electrodeposition process to be studied in close proximity to the substrate in a simple and direct fashion using an inexpensive probe, and without the need for forced convection.
Export citation and abstract BibTeX RIS
The replacement of vacuum-deposited aluminum by plated copper, using the damascene process as a silicon chip interconnect material,1 has reinvigorated investigation into copper metal electrodeposition. Several techniques have been employed in this area to study the effect of plating bath additives such as chloride, polyethylene glycol, thiourea, disulfides, and Janus Green B.2–7 The rotating disk electrode (RDE) method is routinely employed in electroplating studies. However, it has been identified in the literature that the bulky size of a RDE raises technical challenges in incorporating this apparatus into an industrial plating bath,8 as does the need to employ forced convection. Recently microelectrodes were proposed to replace RDEs for in situ monitoring of the influence of additives in copper electroplating baths.8
The RDE approach mentioned above is effective for estimating the amount of additives to be supplied to the electroplating bath to keep the process working at an optimum level. However, more detailed mechanistic information can be attained by employing a rotating ring disk electrode (RRDE). One of the first outcomes derived from investigations that utilized this technique was the detection of  as an intermediate at the disk electrode during the course of the reduction of
as an intermediate at the disk electrode during the course of the reduction of  to Cu metal in a
to Cu metal in a  solution.9, 10 A logical extension of the RRDE technique, using a microelectrode approach, is the scanning electrochemical microscopy (SECM) technique in the substrate generation–tip collection (SG-TC) mode.11 This approach has proved advantageous in studies of redox reactions that take place at solid–solution interfaces because the ultramicroelectrode (UME) probe can be positioned in the solution phase, but in close proximity to a substrate, and hence be used to monitor electroactive species generated in the vicinity of the substrate during the course of an electrochemical experiment.
solution.9, 10 A logical extension of the RRDE technique, using a microelectrode approach, is the scanning electrochemical microscopy (SECM) technique in the substrate generation–tip collection (SG-TC) mode.11 This approach has proved advantageous in studies of redox reactions that take place at solid–solution interfaces because the ultramicroelectrode (UME) probe can be positioned in the solution phase, but in close proximity to a substrate, and hence be used to monitor electroactive species generated in the vicinity of the substrate during the course of an electrochemical experiment.
The SG-TC mode has been used for studying ion transport as a result of charge neutralization during the oxidation/reduction of conducting polymers,12–16 pH changes in the vicinity of a platinum electrode,17 surface oxide formation/reduction on platinum,18 and the flux of electroactive species entering or leaving a Nafion film.19, 20 This method has also been employed to detect transitory species generated during borohydride oxidation at a gold electrode.21 In each of these applications, the UME is held at a potential chosen solely for the purpose of detection of the ion of interest, as opposed to experiments where the UME directly perturbs or chemically alters the sample substrate. It can be readily appreciated that the SG-TC technique is analogous to a RRDE experiment in the sense that the ring electrode is the equivalent of the UME. The advantage of the SECM approach is that processes of interest can be studied without the need for forced convection, as required for RRDE studies. In this study, the SG-TC mode of the SECM technique is employed to confirm that  species can be readily detected at a UME placed in close proximity to glassy carbon (GC) and Pt electrode substrates used for Cu metal deposition from aqueous
species can be readily detected at a UME placed in close proximity to glassy carbon (GC) and Pt electrode substrates used for Cu metal deposition from aqueous  solutions. The implication is that the SECM method offers an attractive alternative means of monitoring metal deposition under conditions relevant to industrial electroplating processes, but without the need to introduce the high forced convection rates required when using the RRDE approach. In addition, to study electrodeposition onto new materials, RDEs or RRDEs need to be constructed from these same materials, which will often be extremely difficult, if not impossible. The SECM-based method negates this requirement.
solutions. The implication is that the SECM method offers an attractive alternative means of monitoring metal deposition under conditions relevant to industrial electroplating processes, but without the need to introduce the high forced convection rates required when using the RRDE approach. In addition, to study electrodeposition onto new materials, RDEs or RRDEs need to be constructed from these same materials, which will often be extremely difficult, if not impossible. The SECM-based method negates this requirement.
It is well known that chloride ions have a stabilizing effect on  ions and also catalyze the rate of copper metal formation from aqueous electrolytes.1, 5, 22–24 Consequently, chloride ions are often introduced into copper electroplating baths. A final feature of the present investigation is to confirm that the catalytic effect of chloride ions may also be conveniently demonstrated by the SECM method.
ions and also catalyze the rate of copper metal formation from aqueous electrolytes.1, 5, 22–24 Consequently, chloride ions are often introduced into copper electroplating baths. A final feature of the present investigation is to confirm that the catalytic effect of chloride ions may also be conveniently demonstrated by the SECM method.
Experimental
Materials and chemicals
Analytical grade  ,
,  , LiCl, NaCl,
, LiCl, NaCl,  ,
,  , and ferrocenemethanol (FcMeOH) were used as received from Aldrich. All aqueous solutions were prepared from water (resistivity
, and ferrocenemethanol (FcMeOH) were used as received from Aldrich. All aqueous solutions were prepared from water (resistivity  ) purified by a Milli-Q reagent deionizer (Millipore Corp.).
) purified by a Milli-Q reagent deionizer (Millipore Corp.).
Procedures
The 3 mm diameter GC (BAS) and 1.5 mm diameter Pt (CH Instruments) substrate electrodes were polished with an aqueous  alumina slurry on a polishing cloth (Microcloth, Buehler), sonicated in deionized water for 5 min, and dried with tissue paper (Kimwipe) prior to use. All solutions were degassed for 10 min with nitrogen prior to undertaking electrochemical experiments.
alumina slurry on a polishing cloth (Microcloth, Buehler), sonicated in deionized water for 5 min, and dried with tissue paper (Kimwipe) prior to use. All solutions were degassed for 10 min with nitrogen prior to undertaking electrochemical experiments.
SECM experiments
Approach curve and imaging experiments were carried out with a CH Instruments model CHI910B scanning electrochemical microscope. The four-electrode configuration of the SG-TC mode consisted of either a GC or Pt substrate electrode, a  diameter Pt UME working electrode (tip electrode with an insulating sheath-to-tip ratio
diameter Pt UME working electrode (tip electrode with an insulating sheath-to-tip ratio  ), a Pt wire counter electrode, and a Ag/AgCl (3 M NaCl) reference electrode. FcMeOH in 0.1 M
), a Pt wire counter electrode, and a Ag/AgCl (3 M NaCl) reference electrode. FcMeOH in 0.1 M  with a known diffusion coefficient of
with a known diffusion coefficient of  was used as a redox mediator to position the UME at a distance of
was used as a redox mediator to position the UME at a distance of  above the substrate electrode, using the approach curve method.11 This mediator solution was then flushed from the cell and replaced with the relevant copper-ion-containing solution used for copper metal electrodeposition experiments.
above the substrate electrode, using the approach curve method.11 This mediator solution was then flushed from the cell and replaced with the relevant copper-ion-containing solution used for copper metal electrodeposition experiments.
Results and Discussion
Copper electrodeposition from acidic media onto a Pt elec trode
The electrodeposition of metallic Cu from an acidified aqueous  solution is often regarded as an ideal nucleation and growth system involving the double-stage electron-transfer reaction scheme shown in Eq. 1, 2
solution is often regarded as an ideal nucleation and growth system involving the double-stage electron-transfer reaction scheme shown in Eq. 1, 2
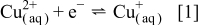
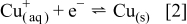
with Eq. 1 being the rate-limiting step. One of the earliest RRDE experiments allowed  to be detected as an intermediate during the course of reduction of
to be detected as an intermediate during the course of reduction of  to Cu in a
to Cu in a  solution.9, 10 This model system therefore was chosen to verify that the SG-TC mode of the SECM method permits even more facile detection of the
solution.9, 10 This model system therefore was chosen to verify that the SG-TC mode of the SECM method permits even more facile detection of the  intermediate in aqueous sulfate electrolyte media.
intermediate in aqueous sulfate electrolyte media.
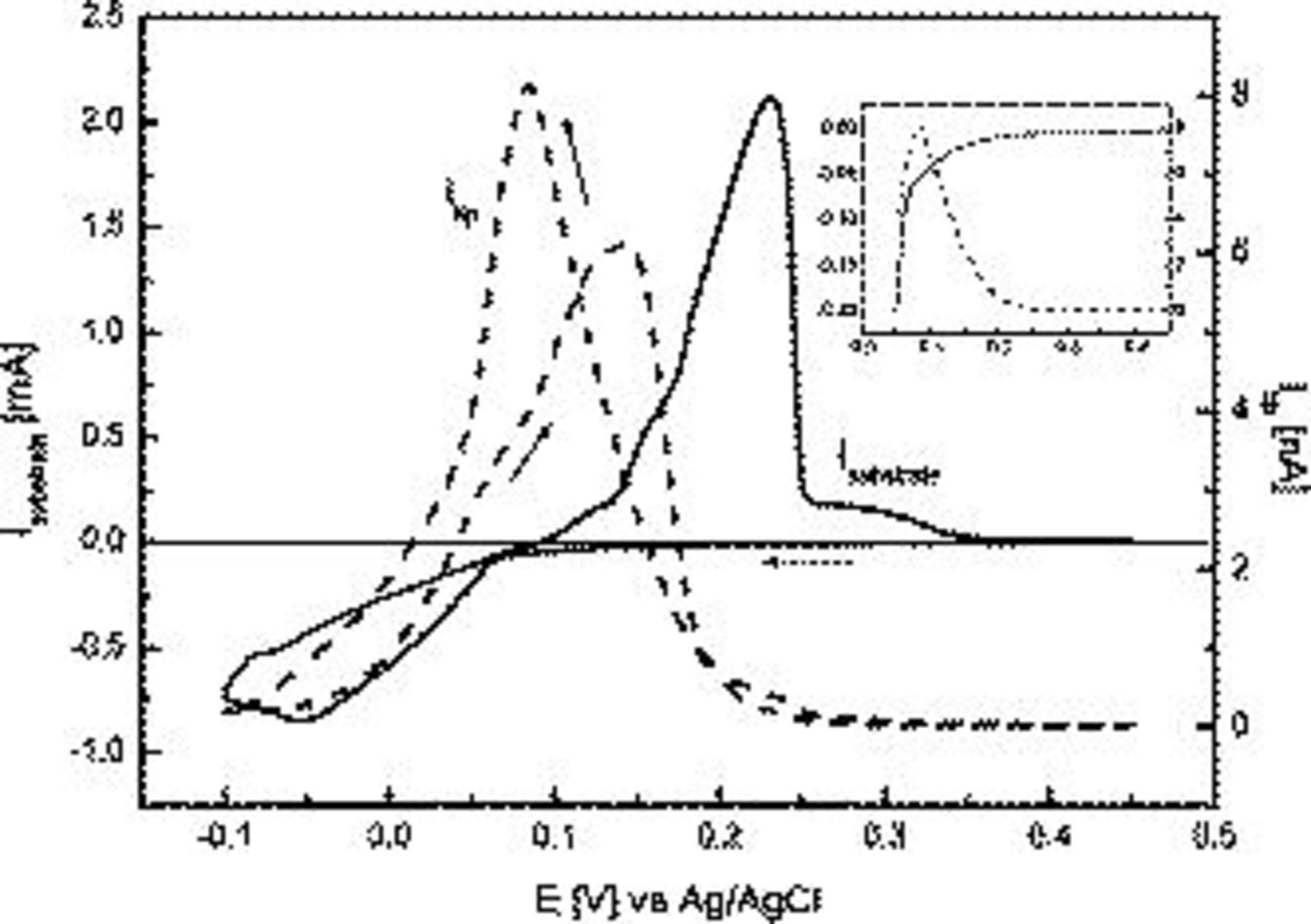
Illustrated in Fig. 1 is a cyclic voltammogram  for the electrodeposition of Cu metal onto a platinum electrode from a 0.1 M
for the electrodeposition of Cu metal onto a platinum electrode from a 0.1 M  –0.5 M
–0.5 M  solution. Upon scanning the potential in the negative direction, the onset of significant reduction current at the substrate Pt electrode is detected clearly at 0.065 V and reaches a maximum value at −0.05 V. The reverse potential sweep direction includes a slight crossover of current occurring at 0.065 V, indicative of nucleation and growth kinetics. Finally, on the reverse sweep, a broad oxidation peak is detected, which is associated with the stripping of Cu metal from the Pt electrode surface. The UME-detected current, also presented in Fig. 1 (designated by
solution. Upon scanning the potential in the negative direction, the onset of significant reduction current at the substrate Pt electrode is detected clearly at 0.065 V and reaches a maximum value at −0.05 V. The reverse potential sweep direction includes a slight crossover of current occurring at 0.065 V, indicative of nucleation and growth kinetics. Finally, on the reverse sweep, a broad oxidation peak is detected, which is associated with the stripping of Cu metal from the Pt electrode surface. The UME-detected current, also presented in Fig. 1 (designated by  ), was recorded at the biased potential of 0.50 V vs Ag/AgCl, allowing only
), was recorded at the biased potential of 0.50 V vs Ag/AgCl, allowing only  , and not
, and not  , to be detected.
, to be detected.  generated at the Pt substrate electrode takes time to diffuse to the UME. Diffusion theory25 provides the relationship between the substrate–tip
generated at the Pt substrate electrode takes time to diffuse to the UME. Diffusion theory25 provides the relationship between the substrate–tip  , the diffusion coefficient
, the diffusion coefficient  , and time (Eq. 3)
, and time (Eq. 3)
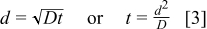
Thus,  takes approximately 0.4 s to diffuse across the substrate–tip gap of
takes approximately 0.4 s to diffuse across the substrate–tip gap of  , assuming a diffusion coefficient value of
, assuming a diffusion coefficient value of  . At a sweep rate of
. At a sweep rate of  and with respect to the tip potential, effectively this gives rise to a −20 mV offset in the forward scan and a +20 mV positive potential offset in the reverse scan, as approximately observed in peak potential values derived from the tip current,
and with respect to the tip potential, effectively this gives rise to a −20 mV offset in the forward scan and a +20 mV positive potential offset in the reverse scan, as approximately observed in peak potential values derived from the tip current,  , vs potential profile in Fig. 1.
, vs potential profile in Fig. 1.
Figure 1. Tip-substrate cyclic voltammograms in 0.1 M  and 0.5 M
and 0.5 M  recorded at
recorded at  at a Pt substrate electrode.
at a Pt substrate electrode.  vs
vs  , and tip-to-substrate
, and tip-to-substrate  . Inset highlights the reduction response over the potential range of 0.45–0.05 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
. Inset highlights the reduction response over the potential range of 0.45–0.05 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
The  response at the UME exhibits an onset of anodic current at 0.23 V (taking the above-mentioned 20 mV shift into consideration), which is well in advance of detection of current
response at the UME exhibits an onset of anodic current at 0.23 V (taking the above-mentioned 20 mV shift into consideration), which is well in advance of detection of current  at 0.065 V, attributed to deposition of Cu at the Pt substrate electrode. However, the amplified inset in Fig. 1 clearly confirms that reduction of
at 0.065 V, attributed to deposition of Cu at the Pt substrate electrode. However, the amplified inset in Fig. 1 clearly confirms that reduction of  actually coincides with an increase in current at the UME, which is assigned to detection of the oxidation of
actually coincides with an increase in current at the UME, which is assigned to detection of the oxidation of  , most likely in the form of the hydrated cuprous ion26
, most likely in the form of the hydrated cuprous ion26  . After initial detection at 0.23 V, the tip current (Fig. 1) increases until it attains a maximum value at a potential of 0.065 V (taking the 20 mV shift into consideration), which coincides with the onset of substantial cathodic current at the substrate. This implies that 0.065 V is the potential where reduction of
. After initial detection at 0.23 V, the tip current (Fig. 1) increases until it attains a maximum value at a potential of 0.065 V (taking the 20 mV shift into consideration), which coincides with the onset of substantial cathodic current at the substrate. This implies that 0.065 V is the potential where reduction of  to Cu metal commences. No
to Cu metal commences. No  is detected at the UME at the switching potential of −0.10 V. In the positive sweep component of the cyclic voltammogram, reduction current continues to be recorded at the substrate until a potential of 0.10 V is reached. The current at the UME over this potential range gradually increases and is attributed to the oxidation of
is detected at the UME at the switching potential of −0.10 V. In the positive sweep component of the cyclic voltammogram, reduction current continues to be recorded at the substrate until a potential of 0.10 V is reached. The current at the UME over this potential range gradually increases and is attributed to the oxidation of  being generated at the substrate (as detected on the forward scan). Over the substrate potential range of 0.10–0.15 V, even though an anodic current is recorded at the substrate, the current at the UME continues to increase. This current at the UME in this potential range is attributed to the oxidation of
being generated at the substrate (as detected on the forward scan). Over the substrate potential range of 0.10–0.15 V, even though an anodic current is recorded at the substrate, the current at the UME continues to increase. This current at the UME in this potential range is attributed to the oxidation of  ions arising from the chemical stripping of Cu metal (nonelectrochemical process) in the presence of
ions arising from the chemical stripping of Cu metal (nonelectrochemical process) in the presence of  ions according to the reaction in Eq. 4
ions according to the reaction in Eq. 4
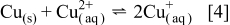
Chemical stripping also has been detected during Cu electrodeposition at a GC substrate using an electrochemical scanning tunneling microscopy (STM) method in which the STM tip was biased to detect  ions in solution.26 In the potential region around 0.15–0.30 V, where a large oxidative Cu stripping peak is located at the substrate electrode, the concentration of
ions in solution.26 In the potential region around 0.15–0.30 V, where a large oxidative Cu stripping peak is located at the substrate electrode, the concentration of  detected at the UME decreases rapidly. This implies that the remaining Cu metal present on the electrode surface is effectively oxidized directly by a two-electron charge-transfer process to yield
detected at the UME decreases rapidly. This implies that the remaining Cu metal present on the electrode surface is effectively oxidized directly by a two-electron charge-transfer process to yield  ions.
ions.
Copper electrodeposition from 0.1 M  onto a GC elec trode
onto a GC elec trode
Figure 2 shows that the cyclic voltammogram for Cu electrodeposition from 0.1 M  (without 0.5 M
(without 0.5 M  present as the added electrolyte) at a GC electrode differs significantly from that observed at a Pt electrode in the acidified
present as the added electrolyte) at a GC electrode differs significantly from that observed at a Pt electrode in the acidified  solution. Even though 0.1 M
solution. Even though 0.1 M  is mildly acidic because of hydrolysis of
is mildly acidic because of hydrolysis of  , migration becomes important in the absence of
, migration becomes important in the absence of  supporting electrolyte. However, it still remains possible to use the response recorded at the UME in the SG-TC mode for detecting transitory species in the absence of added
supporting electrolyte. However, it still remains possible to use the response recorded at the UME in the SG-TC mode for detecting transitory species in the absence of added  supporting electrolyte. Under these much less acidic conditions, and when initially scanning the potential in the negative region, an onset in UME current is detected at 0.30 V (see inset to Fig. 2), indicative of
supporting electrolyte. Under these much less acidic conditions, and when initially scanning the potential in the negative region, an onset in UME current is detected at 0.30 V (see inset to Fig. 2), indicative of  being generated at the GC electrode at about 0.28 V. The maximum UME current is reached at 0.02 V (or about 0.00 V, taking the 20 mV cathodic shift into consideration). As the potential becomes more negative at the GC substrate electrode, the decrease in UME current is attributed to
being generated at the GC electrode at about 0.28 V. The maximum UME current is reached at 0.02 V (or about 0.00 V, taking the 20 mV cathodic shift into consideration). As the potential becomes more negative at the GC substrate electrode, the decrease in UME current is attributed to  consumption at the substrate electrode (as also seen when
consumption at the substrate electrode (as also seen when  is present). Upon reversing the potential scan direction at the GC electrode, the UME current is continuously detected over a wide potential range, even when an anodic current is recorded at the substrate electrode. Again this is consistent with chemical stripping of Cu metal in the presence of
is present). Upon reversing the potential scan direction at the GC electrode, the UME current is continuously detected over a wide potential range, even when an anodic current is recorded at the substrate electrode. Again this is consistent with chemical stripping of Cu metal in the presence of  to produce
to produce  . Finally, upon moving to even more positive potentials at the GC electrode, a gradual decrease in the UME current is detected at potentials where oxidative stripping of Cu metal from GC occurs. This current also is attributed to chemical stripping rather than detection of
. Finally, upon moving to even more positive potentials at the GC electrode, a gradual decrease in the UME current is detected at potentials where oxidative stripping of Cu metal from GC occurs. This current also is attributed to chemical stripping rather than detection of  formed as a long-lived intermediate, during the course of Cu electrochemical oxidation.
formed as a long-lived intermediate, during the course of Cu electrochemical oxidation.
Figure 2. Tip-substrate cyclic voltammograms in 0.1 M  recorded at
recorded at  at a GC substrate electrode.
at a GC substrate electrode.  vs Ag/AgCl, and tip-to-substrate
vs Ag/AgCl, and tip-to-substrate  . Inset highlights the reduction response over the potential range 0.60–0.22 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
. Inset highlights the reduction response over the potential range 0.60–0.22 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
The effect of chloride on Cu electrodeposition
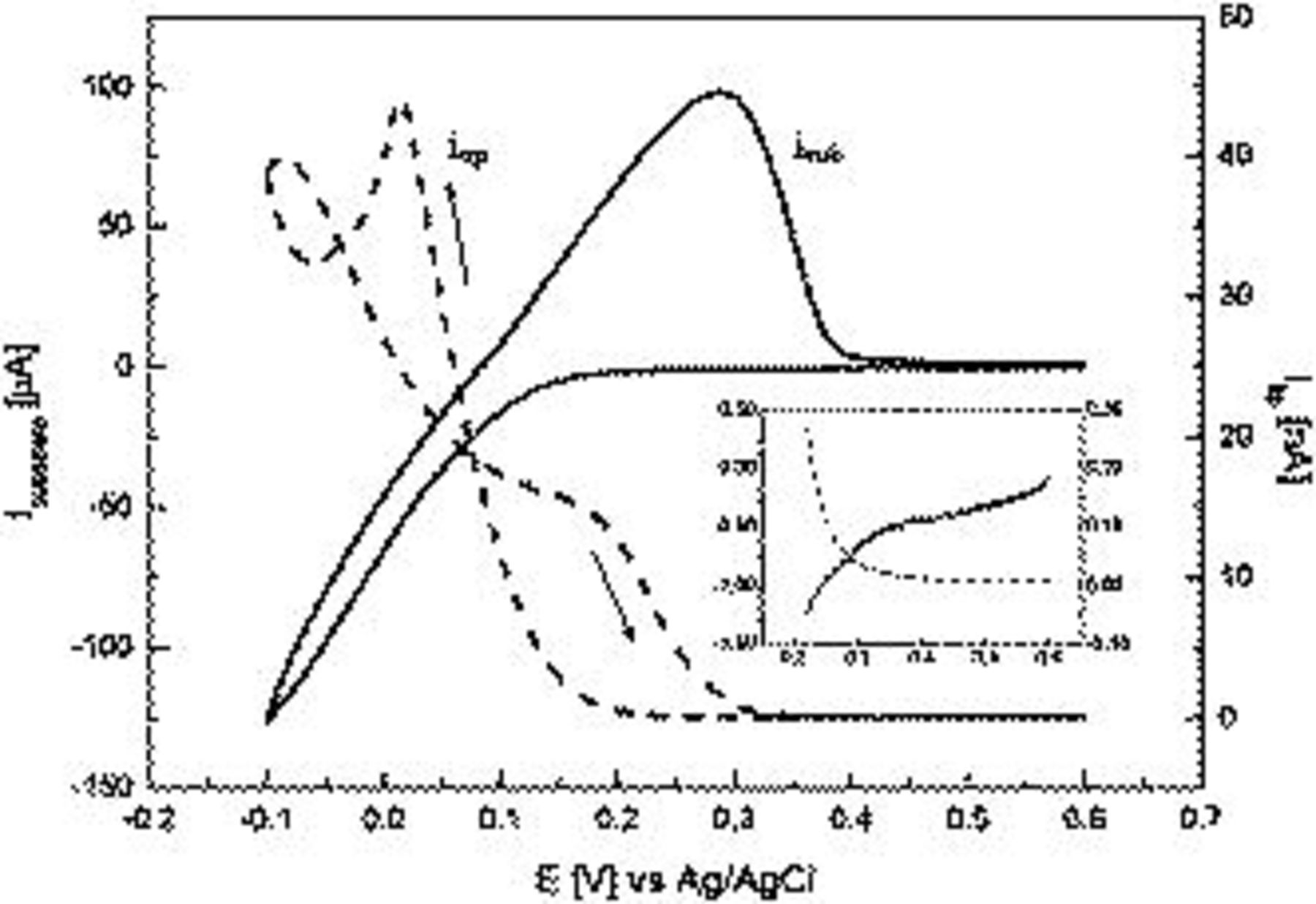
The addition of chloride to copper electroplating baths is well known to both accelerate the reduction of copper ions to copper metal and modify the morphology of the copper deposit.1, 5, 22–24, 27 Figure 3 shows the effect of adding 10 mM NaCl to 0.1 M  during the course of the electrodeposition of Cu at a GC electrode, with SECM monitoring at an UME. The onset potential for Cu deposition in the presence of
during the course of the electrodeposition of Cu at a GC electrode, with SECM monitoring at an UME. The onset potential for Cu deposition in the presence of  now occurs at a more positive potential of 0.32 V (see inset in Fig. 3). This is accompanied by a substantial increase in current being detected at the substrate electrode over the potential range of 0.30–0.00 V. Concomitantly, at the UME, a large (almost 10-fold) increase in current is found at 0.00 V, relative to the case with no chloride present (Fig. 3). It has been speculated that during the course of stepwise reduction of
now occurs at a more positive potential of 0.32 V (see inset in Fig. 3). This is accompanied by a substantial increase in current being detected at the substrate electrode over the potential range of 0.30–0.00 V. Concomitantly, at the UME, a large (almost 10-fold) increase in current is found at 0.00 V, relative to the case with no chloride present (Fig. 3). It has been speculated that during the course of stepwise reduction of  , according to Eq. 1, 2, that the intermediate cuprous ion now complexes with
, according to Eq. 1, 2, that the intermediate cuprous ion now complexes with  to form an insoluble
to form an insoluble  thin layer on the electrode surface which can be reduced at the electrode surface (Eq. 5). However, in the presence of a sufficiently high concentration of
thin layer on the electrode surface which can be reduced at the electrode surface (Eq. 5). However, in the presence of a sufficiently high concentration of  , it may dissolve to form stable
, it may dissolve to form stable  ,1, 5, 24, 28 according to Eq. 6
,1, 5, 24, 28 according to Eq. 6
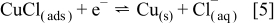
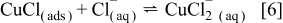
The large increase in current at the GC substrate electrode in the presence of  is attributed to a combination of Reactions 1, 2 (in
is attributed to a combination of Reactions 1, 2 (in  -free solution) and Reaction 5. Given the large increase in current detected at the UME in the presence of
-free solution) and Reaction 5. Given the large increase in current detected at the UME in the presence of  , which is attributed to the oxidation of a
, which is attributed to the oxidation of a  species, it is concluded that a significant concentration of
species, it is concluded that a significant concentration of  is being generated.
is being generated.
Figure 3. Tip-substrate linear sweep voltammograms recorded at  at a GC substrate electrode in 0.1 M
at a GC substrate electrode in 0.1 M  (solid line) and 0.1 M
(solid line) and 0.1 M  containing 10 mM NaCl (dashed line).
containing 10 mM NaCl (dashed line).  vs Ag/AgCl, and tip-to-substrate
vs Ag/AgCl, and tip-to-substrate  . Inset highlights the tip-to-substrate response in 0.1 M
. Inset highlights the tip-to-substrate response in 0.1 M  containing 10 mM NaCl over a potential range of 0.60–0.22 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
containing 10 mM NaCl over a potential range of 0.60–0.22 V but with the same current scale. Units for axes in inset figure are the same as in the main figure.
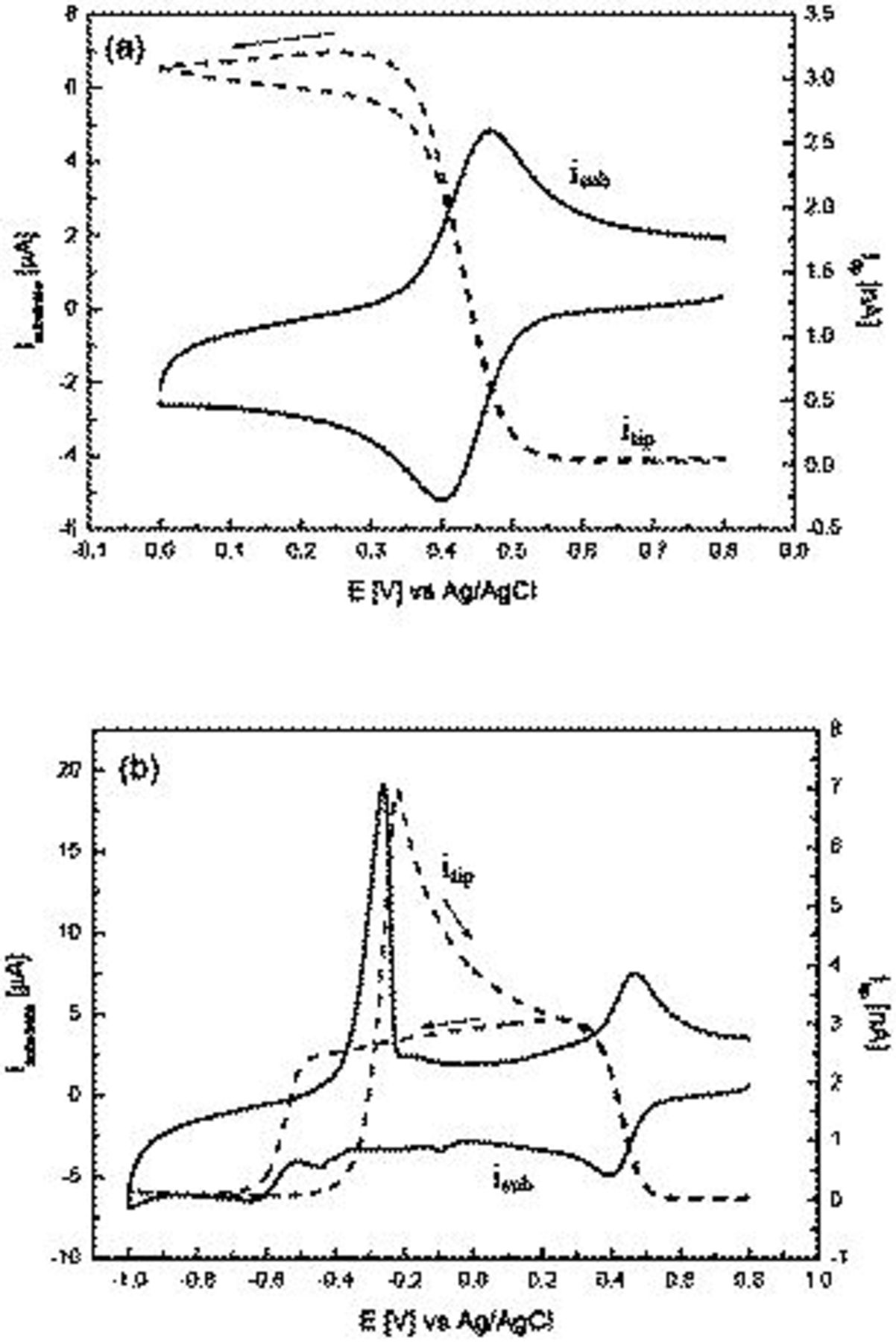
The stability of the cuprous ion can be further enhanced by addition of a large excess of chloride. Figure 4a shows a cyclic voltammogram obtained for the reduction of 1 mM  in the presence of 7 M LiCl as the supporting electrolyte. Under these conditions, a near reversible one-electron (68 mV peak-to-peak separation)
in the presence of 7 M LiCl as the supporting electrolyte. Under these conditions, a near reversible one-electron (68 mV peak-to-peak separation)  couple is observed at 0.43 V. Now, a large increase in UME current (potential held at 0.80 V) is detected at the onset of copper ion reduction at the GC electrode. Upon scanning to more negative potentials at the GC electrode, a near steady-state current is detected at the UME (Fig. 4). In the reverse potential sweep direction, and at potentials where
couple is observed at 0.43 V. Now, a large increase in UME current (potential held at 0.80 V) is detected at the onset of copper ion reduction at the GC electrode. Upon scanning to more negative potentials at the GC electrode, a near steady-state current is detected at the UME (Fig. 4). In the reverse potential sweep direction, and at potentials where  ions are oxidized, a sharp decrease in current at the UME is observed which decays back to zero current by the end of the sweep at 0.80 V. The near steady-state current recorded at the UME in this experiment demonstrates that the electrochemically generated
ions are oxidized, a sharp decrease in current at the UME is observed which decays back to zero current by the end of the sweep at 0.80 V. The near steady-state current recorded at the UME in this experiment demonstrates that the electrochemically generated  ion is highly stable when 7 M LiCl is present.
ion is highly stable when 7 M LiCl is present.
Figure 4. Tip-substrate cyclic voltammograms in 1 mM  and 7 M LiCl recorded at
and 7 M LiCl recorded at  at a GC substrate electrode over a potential range of (a) 0.80–0.00 V and (b) 0.80 to −1.00 V.
at a GC substrate electrode over a potential range of (a) 0.80–0.00 V and (b) 0.80 to −1.00 V.  vs Ag/AgCl, and tip-to-substrate
vs Ag/AgCl, and tip-to-substrate  .
.
Extending the negative switching potential limit in the 7 M LiCl electrolyte case into a region where Cu metal deposition occurs (Fig. 4b) reveals the presence of a second major diffusion-controlled process at the GC electrode at −0.64 V, and two minor processes at −0.10 and −0.45 V. In the reverse sweep to more positive potentials, a clearly defined Cu metal oxidative stripping peak is observed at −0.26 V which regenerates  . At more positive potentials,
. At more positive potentials,  to
to  oxidation occurs. The onset of the
oxidation occurs. The onset of the  process at GC at −0.51 V in the initial negative potential sweep coincides with a sharp decrease in current detected at the UME as in Fig. 4b, which reverted to nearly zero by the end of the sweep at −1.0 V. The behavior for the
process at GC at −0.51 V in the initial negative potential sweep coincides with a sharp decrease in current detected at the UME as in Fig. 4b, which reverted to nearly zero by the end of the sweep at −1.0 V. The behavior for the  reduction is now similar to that observed for the reduction of tetrakis(acetonitrile) copper(I) hexafluorophosphate
reduction is now similar to that observed for the reduction of tetrakis(acetonitrile) copper(I) hexafluorophosphate  in acetonitrile where
in acetonitrile where  exists as a highly stable
exists as a highly stable  complex.29 During the reverse sweep, an increase in UME current is only detected at the onset of the stripping peak at −0.40 V. As the GC electrode potential becomes more positive, a slow decay in UME current occurs until the potential is reached where the
complex.29 During the reverse sweep, an increase in UME current is only detected at the onset of the stripping peak at −0.40 V. As the GC electrode potential becomes more positive, a slow decay in UME current occurs until the potential is reached where the  process is detected at the GC electrode, at which stage a further sharp decrease in current is detected as all of the
process is detected at the GC electrode, at which stage a further sharp decrease in current is detected as all of the  in the vicinity of the electrode is oxidized back to
in the vicinity of the electrode is oxidized back to  . These results clearly illustrate that well-resolved stepwise reduction of
. These results clearly illustrate that well-resolved stepwise reduction of  to
to  to Cu metal occurs in highly concentrated
to Cu metal occurs in highly concentrated  solution. Data also suggest that Cu metal is now oxidized initially to
solution. Data also suggest that Cu metal is now oxidized initially to  . Interestingly, minor features detected at the GC electrode at −0.10 and −0.45 V, which resemble underpotential deposition processes, do not perturb the
. Interestingly, minor features detected at the GC electrode at −0.10 and −0.45 V, which resemble underpotential deposition processes, do not perturb the  oxidation reaction at the UME.
oxidation reaction at the UME.
Conclusions
The SG-TC mode of the SECM can be employed to detect the stepwise reduction of  to
to  and then to Cu metal at GC and Pt electrodes from 0.1 M
and then to Cu metal at GC and Pt electrodes from 0.1 M  solution without and with
solution without and with  and
and  added supporting electrolyte. The catalytic influence of chloride ions on copper electroplating was accompanied by a high concentration of
added supporting electrolyte. The catalytic influence of chloride ions on copper electroplating was accompanied by a high concentration of  being detected during the electrodeposition process. This SECM method opens up the possibility of studying mechanistic aspects of
being detected during the electrodeposition process. This SECM method opens up the possibility of studying mechanistic aspects of  intermediate present during copper electrodeposition in industrial plating bath solutions that contain additives to accelerate Cu metal deposition (including
intermediate present during copper electrodeposition in industrial plating bath solutions that contain additives to accelerate Cu metal deposition (including  ), levelers to produce a smooth deposit, and brighteners under stationary conditions as opposed to use of forced convection required with the RRDE method. This approach is direct and simple and may be carried out without the full SECM apparatus. A manual micrometer stage would suffice. This method also allows electrodeposition studies to be carried out at surfaces where fabrication of RDE or RRDE materials may be difficult.
), levelers to produce a smooth deposit, and brighteners under stationary conditions as opposed to use of forced convection required with the RRDE method. This approach is direct and simple and may be carried out without the full SECM apparatus. A manual micrometer stage would suffice. This method also allows electrodeposition studies to be carried out at surfaces where fabrication of RDE or RRDE materials may be difficult.
All of the experiments carried out in this work were undertaken with macrodisk substrate electrodes (1.5 or 3.0 mm diameter). As noted by Sanchez-Sanchez et al.30 the use of the SG-TC mode with large macrosized substrate electrodes under cyclic voltammetric conditions rules out the possibility of obtaining quantitative kinetic information, as only a small fraction of  generated at the substrate is intercepted by the UME. Alternative approaches are needed to quantify the kinetic chemical steps coupled to charge transfer. Thus, the quantitative determination of hydrogen peroxide during oxygen reduction31 and the kinetics of an electrochemical process (oxidative delamination of
generated at the substrate is intercepted by the UME. Alternative approaches are needed to quantify the kinetic chemical steps coupled to charge transfer. Thus, the quantitative determination of hydrogen peroxide during oxygen reduction31 and the kinetics of an electrochemical process (oxidative delamination of  )32 have been carried out using SECM, but under chronoamperometric conditions. This SECM format allows full account of the feedback effect to be considered at the UME. However, the mechanism of Cu electrodeposition through a stepwise reduction process is extremely complex. It not only involves diffusion processes but also nucleation–growth kinetics at a defect-rich substrate electrode. The analysis of current transients at the UME over a bare and largely uncontrollable Cu-nuclei-covered substrate would be of significant interest but remains exceptionally difficult, even with use of the model described by Sanchez-Sanchez et al.30 Quantification of the kinetics at the RRDE also represents a serious problem.
)32 have been carried out using SECM, but under chronoamperometric conditions. This SECM format allows full account of the feedback effect to be considered at the UME. However, the mechanism of Cu electrodeposition through a stepwise reduction process is extremely complex. It not only involves diffusion processes but also nucleation–growth kinetics at a defect-rich substrate electrode. The analysis of current transients at the UME over a bare and largely uncontrollable Cu-nuclei-covered substrate would be of significant interest but remains exceptionally difficult, even with use of the model described by Sanchez-Sanchez et al.30 Quantification of the kinetics at the RRDE also represents a serious problem.
Acknowledgment
Funding from the Australian Research Council is gratefully acknowledged.