Abstract
A C80 calorimeter was used to study the thermal behaviors of  and
and  in
in 
 electrolyte. C80 results show that
electrolyte. C80 results show that  alone shows one exothermic peak, which is attributed to the solid electrolyte interphase (SEI) decomposition. Four exothermic peaks were detected in
alone shows one exothermic peak, which is attributed to the solid electrolyte interphase (SEI) decomposition. Four exothermic peaks were detected in 
 electrolyte samples. These four peaks are attributed to SEI decomposition, Li-electrolyte reaction as well as new SEI film formation, new SEI film decomposition, and Li with PVDF/other products reactions. The apparent activation energy of
electrolyte samples. These four peaks are attributed to SEI decomposition, Li-electrolyte reaction as well as new SEI film formation, new SEI film decomposition, and Li with PVDF/other products reactions. The apparent activation energy of  and
and  -electrolyte at different states of charge was calculated, and it was found that with intercalated lithium increasing, the activation energy shows a decreasing trend.
-electrolyte at different states of charge was calculated, and it was found that with intercalated lithium increasing, the activation energy shows a decreasing trend.
Export citation and abstract BibTeX RIS
The lithium-ion battery fulfills many of the demands made within the areas of portable electronics and electrical vehicles, and is superior in many ways to the more common nickel-cadmium and nickel-metal hydride batteries.1–5 However, there are also potential safety problems in their use due to the occurrence of thermal runaway in abuse cases.6 The safety is mainly related to the thermal reactivity of the cell materials. The exothermic reactions of materials in battery applications can cause thermal runaway in the cell, and thereby constitute a safety hazard. It has been shown that the thermal stability of anode is critical to the thermal runaway of battery.6–10
Graphite remains the material of choice for anodes in rechargeable lithium-ion batteries because of its high capacity  , flat voltage, and low cost.11 During the first charge, graphite-based electrolytes are reduced at the negative electrode. As a result, a surface film is formed consisting of a variety of solvent and salt reduction products.12, 13 This film functions as an ionic conductor that allows
, flat voltage, and low cost.11 During the first charge, graphite-based electrolytes are reduced at the negative electrode. As a result, a surface film is formed consisting of a variety of solvent and salt reduction products.12, 13 This film functions as an ionic conductor that allows  ion to be transported through the film during the subsequent intercalation and deintercalation processes. The film is also an electronic insulator, which will prevent the continuous reduction of electrolyte as the film thickness reaches a certain limit. The film then functions as a passivating layer on the graphite surface. It is most often referred to as a solid electrolyte interphase (SEI).11–15 Using ac impedance, the formation process of SEI film on graphite electrode during initial cycles was studied.15–19 The results show that the SEI formation takes place through two major stages. The first stage takes place at voltages above
ion to be transported through the film during the subsequent intercalation and deintercalation processes. The film is also an electronic insulator, which will prevent the continuous reduction of electrolyte as the film thickness reaches a certain limit. The film then functions as a passivating layer on the graphite surface. It is most often referred to as a solid electrolyte interphase (SEI).11–15 Using ac impedance, the formation process of SEI film on graphite electrode during initial cycles was studied.15–19 The results show that the SEI formation takes place through two major stages. The first stage takes place at voltages above  (before lithiation of graphite), during which a loose and highly resistive film is formed. The second stage occurs at a narrow voltage range of
(before lithiation of graphite), during which a loose and highly resistive film is formed. The second stage occurs at a narrow voltage range of  , which proceeds simultaneously with lithiation of graphite electrode. In the second stage, a stable, compact, and highly conductive SEI film is produced.11–15
, which proceeds simultaneously with lithiation of graphite electrode. In the second stage, a stable, compact, and highly conductive SEI film is produced.11–15
Studies by means of differential scanning calorimetry (DSC) (Ref. 10, 20–31) and accelerated rate calorimetry (ARC) (Ref. 26, 32–38) have shown that the thermal stability of graphite electrodes is critically dependent on the type of carbon electrode,10, 26, 29, 35 the choice and presence of the electrolyte,20, 22, 39, 40 particularly the lithium salt used in the electrolyte,10, 23, 33, 36, 39–41 state of charge,20, 22, 27, 33 specific surface area,27, 33, 35 binder level in graphite,22, 24, 26, 32 cycle number,27 and so on. The general results have been reviewed by Roth et al. :22 SEI layer decomposition (typically  ); reaction of intercalated lithium with electrolyte solvent
); reaction of intercalated lithium with electrolyte solvent  ; electrolyte decomposition
; electrolyte decomposition  ; positive active material decomposition and reaction with solvent
; positive active material decomposition and reaction with solvent  . Unfortunately, in these studies using DSC, solvent leakage at higher temperatures issue was proposed.22, 25, 27–29, 41 The DSC sample holder started leaking at ca.
. Unfortunately, in these studies using DSC, solvent leakage at higher temperatures issue was proposed.22, 25, 27–29, 41 The DSC sample holder started leaking at ca.  ,29 or
,29 or  ,27 as the sample holders were not designed for a possible gas evolution reaction. Furthermore, ARC is available to research self-heating substances, but is unfit to detect endothermic reactions.42
,27 as the sample holders were not designed for a possible gas evolution reaction. Furthermore, ARC is available to research self-heating substances, but is unfit to detect endothermic reactions.42
The thermal stability of the SEI layer, and the graphite electrode in general, is crucial to its use in practical cells. However, the thermal decomposition kinetics is seldom reported; therefore, a Calvet calorimeter C80, with good airtight characteristics and precision, was used to investigate the thermal stability of lithiation graphite with 
 carbonate (EC)+diethyl carbonate (DEC) electrolyte in detail to disclose their thermal stabilities and thermal decomposition kinetics.
carbonate (EC)+diethyl carbonate (DEC) electrolyte in detail to disclose their thermal stabilities and thermal decomposition kinetics.
Experimental
The graphite electrode used in this study consists of a mixture of graphite (Hongyuan Carbon Industry Co., Ltd) and polyvinylidene fluoride (PVDF) binder. The graphite electrode is made of 92% graphite material and 8% binder, cast on copper foil. The electrodes were obtained ready for use, dried overnight in vacuum at  , and handled in an argon-filled glove box (MBraun Labmaster 130,
, and handled in an argon-filled glove box (MBraun Labmaster 130, 
 and
and  ). The graphite electrode was cut as a
). The graphite electrode was cut as a  diameter disk of about
diameter disk of about  thickness to get more mass of sample for the C80 experiment.
thickness to get more mass of sample for the C80 experiment.
Electrochemical cycling was performed in button-type coin cells (CR 2032). Coin cells were assembled in an argon-filled glove box with the graphite as positive electrode, lithium as counter electrode, 

 as the electrolyte, and Celgard 2400 polyethylene as the separator (
as the electrolyte, and Celgard 2400 polyethylene as the separator ( thickness).
thickness).
The cells were galvanostatically cycled on a multichannel battery cycler (Neware BTS2300, Shenzhen) at  current density in the first three formation cycles. The cells were charged to
current density in the first three formation cycles. The cells were charged to  and then discharged to
and then discharged to  for Li/graphite cell. The definition of "discharge" here is for the half-cells, namely, the lithiation process for the graphite electrodes. If they are used as the anodes in full cells, this process corresponds to the "charge" step. Then, the cells were discharged to different state of discharge for thermal test.
for Li/graphite cell. The definition of "discharge" here is for the half-cells, namely, the lithiation process for the graphite electrodes. If they are used as the anodes in full cells, this process corresponds to the "charge" step. Then, the cells were discharged to different state of discharge for thermal test.
Then, the discharged cells were disassembled in a glove box. To remove the electrolyte from the electrode, the wet charged electrode powder was placed into a bottle. To that bottle a portion of dimethyl carbonate (DMC), a volatile organic solvent, was added and the bottle was then shaken by hand. The sample was then decanted and the DMC rinsing procedure was repeated. After the second decanting, the sample was dried to remove the DMC solvent. As the DMC can be followed by a partial dissolution of the polymeric components of the SEI, which in turn may decrease the integrity and real thermal stability of the SEI, the DMC rinsing procedure is done no more than twice. After drying, the electrode material was scraped, trying not to abrade the SEI film, from the copper current collectors carefully for thermal-testing. To characterize the thermal stability of the lithiated electrodes in the presence of electrolyte, about equal amounts of lithiated electrode material (including PVDF) and 
 electrolyte were transferred into a high-pressure stainless steel vessel (
electrolyte were transferred into a high-pressure stainless steel vessel ( in volume) of a microcalorimeter (Setaram C80) sealed in argon atmosphere. The weight of each sample
in volume) of a microcalorimeter (Setaram C80) sealed in argon atmosphere. The weight of each sample  was measured before and after the experiment to verify that the system was hermetically sealed. The weight was constant in all cases, indicating that there were no leaks during the experiments. The measurements were carried out using a heating rate set at
was measured before and after the experiment to verify that the system was hermetically sealed. The weight was constant in all cases, indicating that there were no leaks during the experiments. The measurements were carried out using a heating rate set at  in the temperature range
in the temperature range  . The thermal effects of each sample with temperature were thus recorded automatically, and the C80 calculations were based on dry film weight of the electrode material.
. The thermal effects of each sample with temperature were thus recorded automatically, and the C80 calculations were based on dry film weight of the electrode material.
Results and Discussion
Cycling profile of a graphite anode
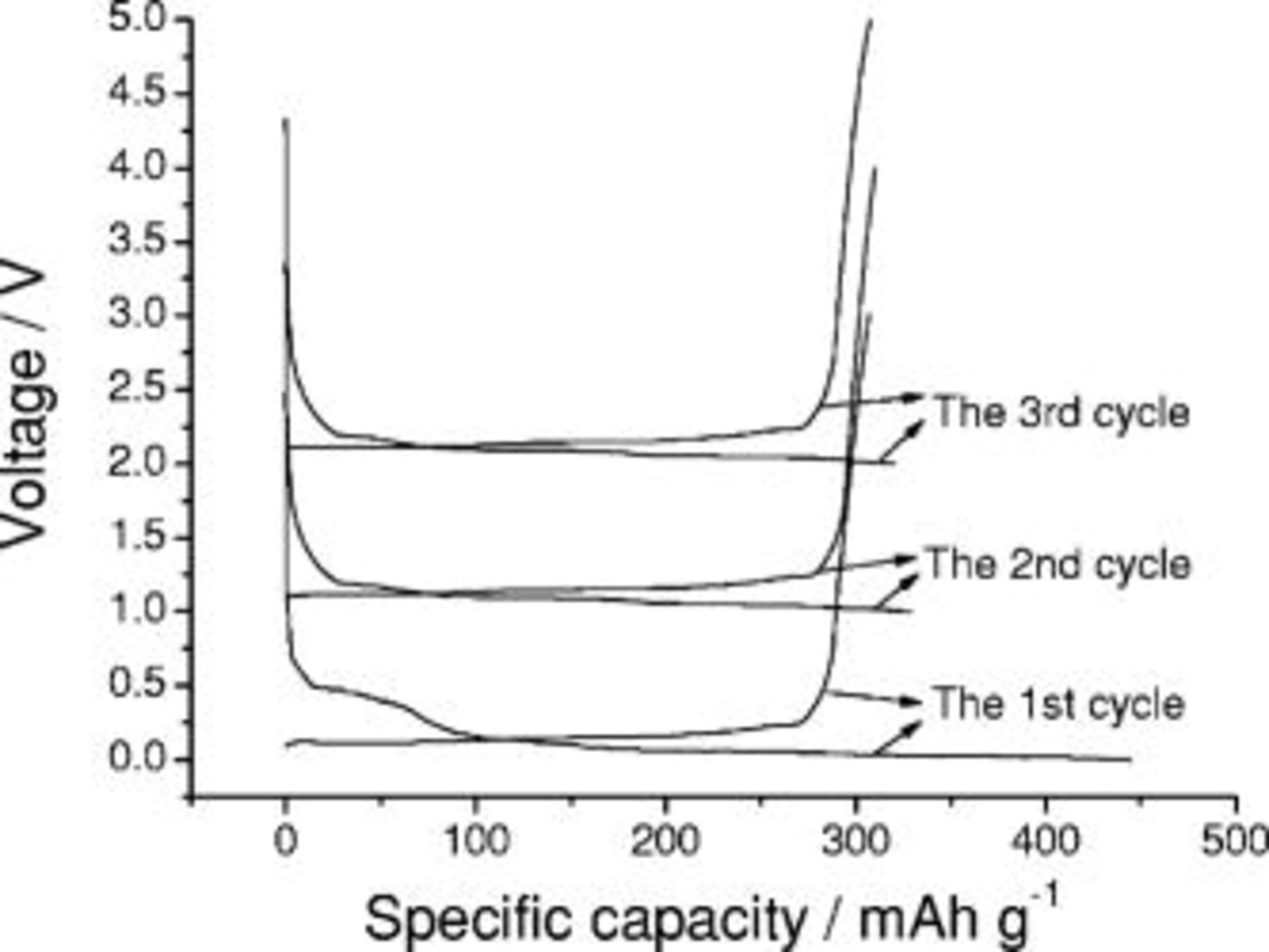
Figure 1 shows the first three cycling profiles of a graphite anode with a lithium foil as a counter electrode at a current of  . A plateau observed near
. A plateau observed near  in the first discharge process is associated with the decomposition of the electrolyte on the surface of graphite in the formation of the passivating layer.12, 13, 28 This plateau disappears in subsequent cycling because the passivating layer suppresses further electrolyte decomposition. The irreversible capacity observed in the first three cycles for graphite electrode are 138, 19, and
in the first discharge process is associated with the decomposition of the electrolyte on the surface of graphite in the formation of the passivating layer.12, 13, 28 This plateau disappears in subsequent cycling because the passivating layer suppresses further electrolyte decomposition. The irreversible capacity observed in the first three cycles for graphite electrode are 138, 19, and  , respectively, suggesting that after the first three cycles, the formatted SEI film has been formed.
, respectively, suggesting that after the first three cycles, the formatted SEI film has been formed.
Figure 1. The cycling profile of a graphite anode containing PVDF-binder with a lithium foil as a counter electrode.
Typical thermal behavior profiles
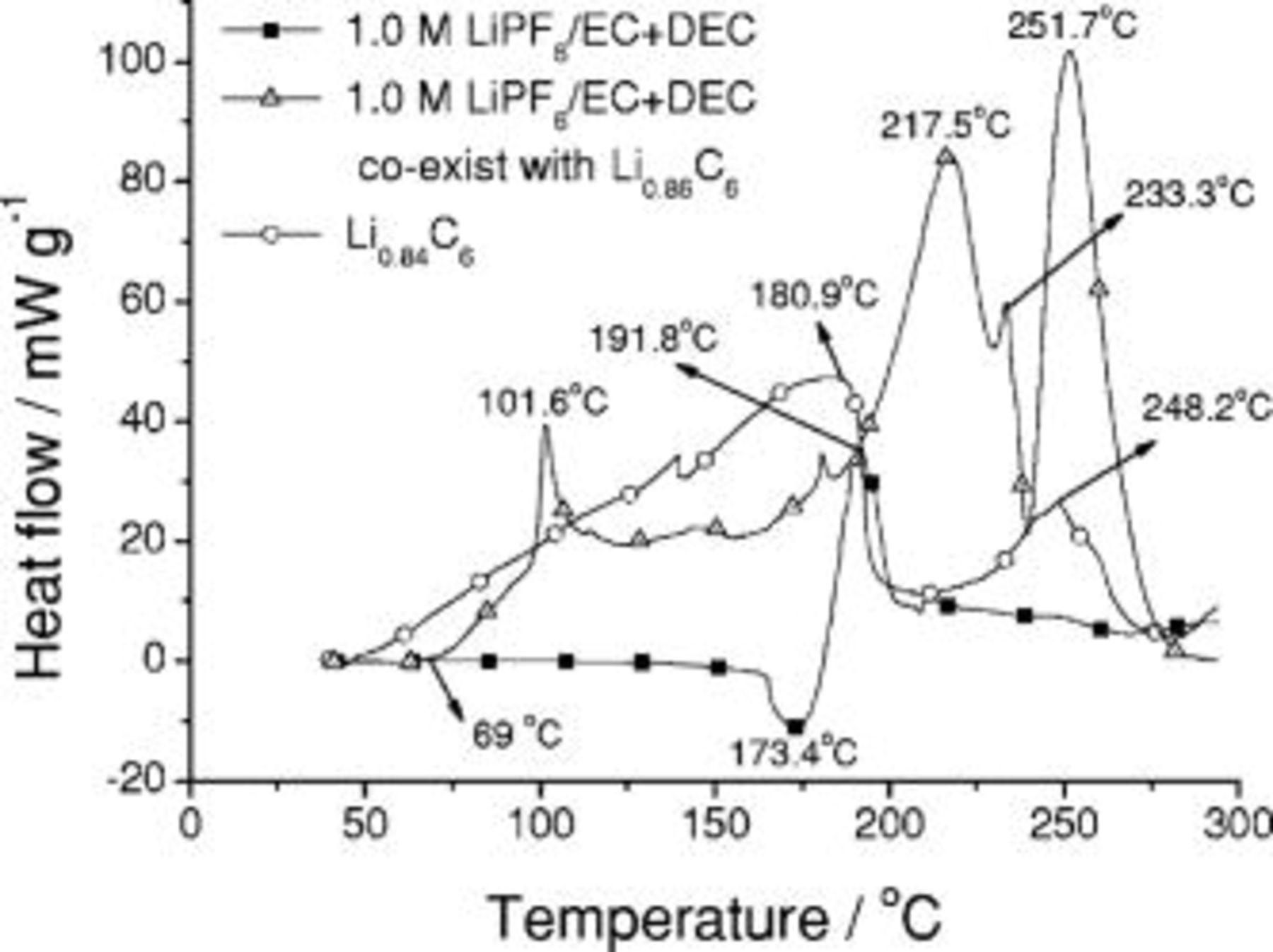
Figure 2 presents the C80 heat flow curves obtained from the rinsed lithiated graphite anode sample, 
 electrolyte and their coexisting system. This figure shows that the rinsed lithiated graphite, i.e.,
electrolyte and their coexisting system. This figure shows that the rinsed lithiated graphite, i.e.,  , starts to be exothermic at about
, starts to be exothermic at about  , and reaches the first peak at
, and reaches the first peak at  , the mild second peak at
, the mild second peak at  with the total heat generation of
with the total heat generation of  . An endothermic peak at
. An endothermic peak at  and an exothermic peak at
and an exothermic peak at  are detected in the C80 scan of
are detected in the C80 scan of 
 electrolyte. Three obvious exothermic peaks are detected at 102, 218, and
electrolyte. Three obvious exothermic peaks are detected at 102, 218, and  , respectively, with total heat generation of
, respectively, with total heat generation of  (based on
(based on  ) in the C80 experiment of
) in the C80 experiment of  coexisting with
coexisting with 
 electrolyte.
electrolyte.
Figure 2. C80 heat flow curves of lithiated graphite  ,
, 
 electrolyte, and
electrolyte, and  -electrolyte at a heating rate of
-electrolyte at a heating rate of  .
.
The rinsed lithiated graphite contains graphite, intercalated lithium, SEI layer, and PVDF. The SEI layer mainly consists of stable (such as LiF,  ), and metastable components [such as polymers,
), and metastable components [such as polymers,  ,
,  , and ROLi].6, 20, 21, 28, 33 The graphite, LiF, and
, and ROLi].6, 20, 21, 28, 33 The graphite, LiF, and  are stable, and they do not decompose and produce heat below
are stable, and they do not decompose and produce heat below  . The PVDF has a minimal effect on the thermal response of the anode reactions.22 Therefore, the peak of
. The PVDF has a minimal effect on the thermal response of the anode reactions.22 Therefore, the peak of  in C80 experiment is attributed to the SEI breakdown and its reaction with intercalated lithium as21, 28, 33
in C80 experiment is attributed to the SEI breakdown and its reaction with intercalated lithium as21, 28, 33


Assuming the reaction mechanism is dependent on the Arrhenius law, based on the C80 data, the following equation 3 is obtained as43, 44

where  is over all heat flow,
is over all heat flow,  is heat of reaction,
is heat of reaction,  is initial mass of reactant,
is initial mass of reactant,  is activation energy,
is activation energy,  is gas constant,
is gas constant,  is temperature of system, and
is temperature of system, and  is pre-exponential factor. By plotting the curve of
is pre-exponential factor. By plotting the curve of  versus inverse temperature, the
versus inverse temperature, the  and
and  can be easily calculated.43 By this method, the SEI thermal decomposition activation energy is calculated as
can be easily calculated.43 By this method, the SEI thermal decomposition activation energy is calculated as  , and pre-exponential factor is
, and pre-exponential factor is  .
.
With the presence of electrolyte together with  , three exothermic peaks are detected in the C80 experiment. This coexisting system starts to decompose at
, three exothermic peaks are detected in the C80 experiment. This coexisting system starts to decompose at  , and reaches the first peak at
, and reaches the first peak at  with heat generation of
with heat generation of  , which is earlier than the rinsed
, which is earlier than the rinsed  alone. This first exothermic process should be the thermal decomposition of SEI layer judging from previous studies. It is reported23, 28 that
alone. This first exothermic process should be the thermal decomposition of SEI layer judging from previous studies. It is reported23, 28 that  was the major species responsible for the destruction of the SEI layer at high temperatures. Because
was the major species responsible for the destruction of the SEI layer at high temperatures. Because  is a powerful Lewis acid, which is produced by the equilibrium decomposition
is a powerful Lewis acid, which is produced by the equilibrium decomposition  , and attacks the electron lone pair or the atom with a large electron density, it is likely to react with the oxygen atom of carbonyl groups
, and attacks the electron lone pair or the atom with a large electron density, it is likely to react with the oxygen atom of carbonyl groups  and compounds possessing sites of increased electron density. In addition, the dominant component of the SEI is
and compounds possessing sites of increased electron density. In addition, the dominant component of the SEI is  , indicating the C–O functional group. Hence, it is reasonable to propose that
, indicating the C–O functional group. Hence, it is reasonable to propose that  damages the SEI in a
damages the SEI in a  system at
system at  .23, 28 Using the above method, the activation energy is calculated as
.23, 28 Using the above method, the activation energy is calculated as  , and the pre-exponential factor is
, and the pre-exponential factor is  .
.
The breakdown of SEI layer makes the electrolyte easy to reach the lithiated graphite surface, furthermore, the intercalated Li ions move from the inner structure to the edge of graphite particles at high temperatures,23, 45 and  accelerates the Li movement by removing electrons from graphite. Then, the intercalated lithium easily reacts with electrolyte directly. A mild heat generation continues from
accelerates the Li movement by removing electrons from graphite. Then, the intercalated lithium easily reacts with electrolyte directly. A mild heat generation continues from  until an exothermic peak appears at
until an exothermic peak appears at  in C80 experiment of
in C80 experiment of  -electrolyte in Fig. 2. This peak is attributed to the reaction of lithiated graphite with the electrolyte.24, 46 Several studies have concluded that intercalated lithium reacts with the electrolyte to form a new stable SEI film after SEI decomposition at the edge planes.24, 27, 28, 33 The formation of secondary SEI film reactions stops when either the new film is thick enough to insulate reactants or all the intercalated Li has been consumed. This new SEI film prevents the electrolyte from continued reaction with intercalated lithium. As this new SEI film could decompose with rising temperature, therefore, the peak at
-electrolyte in Fig. 2. This peak is attributed to the reaction of lithiated graphite with the electrolyte.24, 46 Several studies have concluded that intercalated lithium reacts with the electrolyte to form a new stable SEI film after SEI decomposition at the edge planes.24, 27, 28, 33 The formation of secondary SEI film reactions stops when either the new film is thick enough to insulate reactants or all the intercalated Li has been consumed. This new SEI film prevents the electrolyte from continued reaction with intercalated lithium. As this new SEI film could decompose with rising temperature, therefore, the peak at  is contributed by new SEI film decomposition process. ARC results35 disclosed that between 90 and
is contributed by new SEI film decomposition process. ARC results35 disclosed that between 90 and  ,
,  and EC/DEC react to form Li-alkyl carbonates, which are consistent with the coupled earlier exothermic processes in our experiments. It is reported22, 28 that PVDF has a minimal effect on the thermal response of the anode reactions; then, the peak at
and EC/DEC react to form Li-alkyl carbonates, which are consistent with the coupled earlier exothermic processes in our experiments. It is reported22, 28 that PVDF has a minimal effect on the thermal response of the anode reactions; then, the peak at  is attributed to overlapped reactions of PVDF,
is attributed to overlapped reactions of PVDF,  , Li-alkyl carbonates, and EC/DEC with the formation
, Li-alkyl carbonates, and EC/DEC with the formation  . Some researchers24, 26, 27 attributed this peak to the reaction of lithiated graphite with PVDF, Yamaki et al.24 suggested that the sharp exothermic peak at
. Some researchers24, 26, 27 attributed this peak to the reaction of lithiated graphite with PVDF, Yamaki et al.24 suggested that the sharp exothermic peak at  (corresponding to the peak at
(corresponding to the peak at  in our experiments, as the heating rate,
in our experiments, as the heating rate,  , is lower than Yamaki's,
, is lower than Yamaki's,  ) was the direct reaction of the lithiated graphite with electrolyte due to SEI breakdown. We believe that the salts and/or EC/DEC are also involved in these reactions.28 The reaction heat of intercalated lithium with electrolyte (second exothermic process) is
) was the direct reaction of the lithiated graphite with electrolyte due to SEI breakdown. We believe that the salts and/or EC/DEC are also involved in these reactions.28 The reaction heat of intercalated lithium with electrolyte (second exothermic process) is  , and the activation energy is calculated as
, and the activation energy is calculated as  , and pre-exponential factor is
, and pre-exponential factor is  .
.
 and
and  -electrolyte thermal behavior
-electrolyte thermal behavior
Figure 3 presents the C80 heat flow curves obtained from lithiated graphite anode samples at a  heating rate from
heating rate from  . One main exothermic peak is detected in each sample. With the amount of intercalated lithium increasing in the graphite, the onset temperature presents a decreasing trend; however, the exothermic peak swing instead of adhering strictly to this decreasing trend. At
. One main exothermic peak is detected in each sample. With the amount of intercalated lithium increasing in the graphite, the onset temperature presents a decreasing trend; however, the exothermic peak swing instead of adhering strictly to this decreasing trend. At  , 0.52, and 0.76, small exothermic process presents before the main large exothermic peaks. These processes are according to the SEI decomposition in theirs coexisting system; at
, 0.52, and 0.76, small exothermic process presents before the main large exothermic peaks. These processes are according to the SEI decomposition in theirs coexisting system; at  , a small peak at
, a small peak at  is observed too. In other situations, these processes are absent or not obvious. However, the exothermic curves are not smooth, which may be the overlapped results of different reactions, including SEI decomposition. These may be attributed to the effect of DMC, as DMC may dissolve parts of the organic SEI film. It is suggested that the prewashing process damages the SEI by dissolving some of its constituents (such as polymers and semicarbonates) and causing a fast vigorous reaction.20 The heat generation presents an increasing trend, which is clearly exhibited in Fig. 4. Yang et al.28 have proposed that the peak associated with the SEI film decomposition increases with the increasing intercalated lithium, but this trend is not obvious in their experiments. The heat generation of
is observed too. In other situations, these processes are absent or not obvious. However, the exothermic curves are not smooth, which may be the overlapped results of different reactions, including SEI decomposition. These may be attributed to the effect of DMC, as DMC may dissolve parts of the organic SEI film. It is suggested that the prewashing process damages the SEI by dissolving some of its constituents (such as polymers and semicarbonates) and causing a fast vigorous reaction.20 The heat generation presents an increasing trend, which is clearly exhibited in Fig. 4. Yang et al.28 have proposed that the peak associated with the SEI film decomposition increases with the increasing intercalated lithium, but this trend is not obvious in their experiments. The heat generation of  and decomposition dynamics parameters were calculated in Table I.
and decomposition dynamics parameters were calculated in Table I.
Figure 3. C80 heat flow curves of graphite sample with different amount of intercalated lithium at a heating rate of  . In
. In  ,
,  denotes the level of intercalation.
denotes the level of intercalation.
Figure 4. Onset temperature, exothermic peak, and overall heat generation of graphite samples with different amounts of intercalated Li.
Table I. The thermal characteristics and decomposition dynamics parameters of  .
.
 in in 
| Onset temperature (°C) | Peak (°C) | Heat generation ( ) ) | Activation energy ( ) ) | Pre-exponential factor A( ) ) |
|---|---|---|---|---|---|
| 0.18 | 136 | 207 |

| 115.3 |

|
| 0.22 | 65 | 247 |

| 103.8 |

|
| 0.32 | 47 | 204 |

| 105.8 |

|
| 0.45 | 50 | 166 |

| 86.6 |

|
| 0.52 | 44 | 213 |

| 96.5 |

|
| 0.68 | 42 | 219 |

| 68.8 |

|
| 0.76 | 41 | 234 |

| 82.0 |

|
| 0.84 | 47 | 181 |

| 101.3 |

|
| 0.92 | 42 | 224 |

| 77.0 |

|
With the presence of 
 electrolyte in the lithiated graphite, in Fig. 5 all the samples show similar thermal behavior at elevated temperature. Obvious peaks can be identified and are designated peaks
electrolyte in the lithiated graphite, in Fig. 5 all the samples show similar thermal behavior at elevated temperature. Obvious peaks can be identified and are designated peaks  ,
,  , and
, and  in the text. A faint peak between peak
in the text. A faint peak between peak  and peak
and peak  appears uncertain; it is coupled with other process and designated peak
appears uncertain; it is coupled with other process and designated peak  for unification. Judging from the above analysis,
for unification. Judging from the above analysis,  ,
,  ,
,  , and
, and  peaks are attributed to the SEI breakdown, lithium-electrolyte reaction, new SEI film breakdown, and
peaks are attributed to the SEI breakdown, lithium-electrolyte reaction, new SEI film breakdown, and  formation reactions overlapped with PVDF reactions, respectively. The SEI breakdown peaks-remain around
formation reactions overlapped with PVDF reactions, respectively. The SEI breakdown peaks-remain around  because the SEI layer's main compositions, LiF,
because the SEI layer's main compositions, LiF,  ,
,  ,
,  , and ROLi, have been formed in the first cycle;20, 33, 45 therefore, its thermal stability is not influenced by the intercalated degree of lithium. After the breakdown of former SEI layer, the reaction peak
, and ROLi, have been formed in the first cycle;20, 33, 45 therefore, its thermal stability is not influenced by the intercalated degree of lithium. After the breakdown of former SEI layer, the reaction peak  , around
, around  , of electrolyte with intercalated lithium presents little increasing trend with intercalated lithium increasing. Peak
, of electrolyte with intercalated lithium presents little increasing trend with intercalated lithium increasing. Peak  is the breakdown of secondary SEI, and presents increasing trend of heat generation with the increasing intercalated degree of lithium. The breakdown of secondary SEI film permits the rest of the intercalated lithium to react with the electrolyte or its decomposition products, as the electrolyte decomposes below
is the breakdown of secondary SEI, and presents increasing trend of heat generation with the increasing intercalated degree of lithium. The breakdown of secondary SEI film permits the rest of the intercalated lithium to react with the electrolyte or its decomposition products, as the electrolyte decomposes below  (Fig. 2). The electrolyte decomposition products are
(Fig. 2). The electrolyte decomposition products are  ,
,  ,
,  , HF,
, HF,  ,
,  , etc.47, 48 Then, the intercalated lithium will react with these products, which results in the peaks
, etc.47, 48 Then, the intercalated lithium will react with these products, which results in the peaks  in Fig. 5. After
in Fig. 5. After  , peaks
, peaks  become more sharp (Yang et al.23 reported a sharper peak at
become more sharp (Yang et al.23 reported a sharper peak at  using DSC at a
using DSC at a  heating rate); this sharper peak corresponds to the peaks of
heating rate); this sharper peak corresponds to the peaks of  at
at  heating rate. Aurbach et al.49 and Chung et al.50 noted that EC and
heating rate. Aurbach et al.49 and Chung et al.50 noted that EC and  can enter into the graphite sheets and react with intercalated lithium to form lithium carbonate and ethylene. This process is similar to the exfoliation in the first cycle of Li intercalation and can bring about significant changes in the surface structure, including the breakdown of the graphite powder into fragments. Therefore, the sharp peak
can enter into the graphite sheets and react with intercalated lithium to form lithium carbonate and ethylene. This process is similar to the exfoliation in the first cycle of Li intercalation and can bring about significant changes in the surface structure, including the breakdown of the graphite powder into fragments. Therefore, the sharp peak  detected for
detected for  -electrolyte samples in Fig. 5 is partly attributed to the collapse of the graphite structure.28 The released Li from the collapsed graphite particles can react with PVDF and electrolyte decomposition products at this high temperature and produce more heat. These reactions can be attributed to the dehydrofluorination of PVDF and formation of LiF and hydrogen, and so on.
-electrolyte samples in Fig. 5 is partly attributed to the collapse of the graphite structure.28 The released Li from the collapsed graphite particles can react with PVDF and electrolyte decomposition products at this high temperature and produce more heat. These reactions can be attributed to the dehydrofluorination of PVDF and formation of LiF and hydrogen, and so on.
Figure 5. C80 heat flow curves of  with the presence of equal amount
with the presence of equal amount 
 electrolyte at a heating rate of
electrolyte at a heating rate of  . In
. In  ,
,  denotes the level of intercalation.
denotes the level of intercalation.
The thermal parameters and apparent activation energy were summarized in Table II. The activation energy of SEI decomposition (peak  ) and Li-electrolyte reaction (peak
) and Li-electrolyte reaction (peak  ) was determined based on Arrhenius laws. The activation energy of SEI decomposition without much deviation with
) was determined based on Arrhenius laws. The activation energy of SEI decomposition without much deviation with  changing, which indicates the SEI is a stabilized unit formed by LiF, Li2CO3, ROCO2LI, (CH2OCO2Li)2, and ROLi. The apparent activation energy of Li-electrolyte reaction is without much changing too, possibly explained as follows. Before
changing, which indicates the SEI is a stabilized unit formed by LiF, Li2CO3, ROCO2LI, (CH2OCO2Li)2, and ROLi. The apparent activation energy of Li-electrolyte reaction is without much changing too, possibly explained as follows. Before  , Li is consumed completely before the new SEI film completely blocks the surface of edge planes, as Li is very active in high temperature; therefore, the more the Li in
, Li is consumed completely before the new SEI film completely blocks the surface of edge planes, as Li is very active in high temperature; therefore, the more the Li in  the lower the activation energy, and no sharp graphite structure collapse was detected in the last exothermic process in
the lower the activation energy, and no sharp graphite structure collapse was detected in the last exothermic process in  -electrolyte samples. At
-electrolyte samples. At  , the new SEI film prevents the Li from reacting with electrolyte, and there about 0.08 intercalated lithium per
, the new SEI film prevents the Li from reacting with electrolyte, and there about 0.08 intercalated lithium per  stays in the graphite sheet, which is not sufficient to generate a sharp peak from the reaction with PVDF and electrolyte decomposition by-products after the new SEI film breakdown. After
stays in the graphite sheet, which is not sufficient to generate a sharp peak from the reaction with PVDF and electrolyte decomposition by-products after the new SEI film breakdown. After  , the more intercalated Li (
, the more intercalated Li ( per
per  ) remaining in the graphite, the more intercalated lithium remaining renders the structure more unstable and results in lower activation energy for this structural collapse reaction, which occurs at a lower temperature. The released lithium as a result of the structural collapse reacts with the PVDF binder and electrolyte decomposition by-products to form LiF, hydrogen, and
) remaining in the graphite, the more intercalated lithium remaining renders the structure more unstable and results in lower activation energy for this structural collapse reaction, which occurs at a lower temperature. The released lithium as a result of the structural collapse reacts with the PVDF binder and electrolyte decomposition by-products to form LiF, hydrogen, and  .
.
Table II. The thermal characteristics and decomposition dynamics parameters of 
 electrolyte.
electrolyte.
 in in

| Onset temperature (°C) | Peaks (°C) | Heat generation ( ) ) | Total | Activation energy  ( ( ) ) | Pre-exponential factor A( ) ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|

|

|

|

|

|

|

|

|

|

|

| |||
| 0.19 | 61 | 94 | 211 | 228 | 251 |

|

|

|

|

| 100.4 | 93.0 |

|

|
| 0.22 | 67 | 96 | 204 | 224 | 246 |

|

|

|

|

| 115.2 | 81.2 |

|

|
| 0.38 | 59 | 102 | 210 | 228 | 241 |

|

|

|

|

| 77.4 | 62.4 |

|

|
| 0.46 | 57 | 100 | 211 | 223 | 242 |

|

|

|

|

| 92.8 | 83.0 |

|

|
| 0.52 | 51 | 103 | 216 | 235 | 247 |

|

|

|

|

| 83.7 | 62.5 |

|

|
| 0.61 | 64 | 100 | 216 | 231 | 243 |

|

|

|

|

| 99.1 | 78.0 |

|

|
| 0.73 | 58 | 99 | 214 | 228 | 243 |

|

|

|

|

| 83.8 | 68.4 |

|

|
| 0.86 | 69 | 102 | 218 | 233 | 252 |

|

|

|

|

| 78.6 | 64.3 |

|

|
| 0.92 | 59 | 101 | 217 | 234 | 249 |

|

|

|

|

| 96.2 | 69.1 |

|

|
Conclusions
Thermal behaviors of the graphite anode with/without electrolyte at different states of charge are studied using C80 orimeter at a  heating rate in the temperature range
heating rate in the temperature range  . The rinsed
. The rinsed  alone shows one exothermic peak, which is attributed to the SEI decomposition and intercalated lithium reacting with SEI compounds. With the presence of an equal amount of
alone shows one exothermic peak, which is attributed to the SEI decomposition and intercalated lithium reacting with SEI compounds. With the presence of an equal amount of 
 electrolyte in the
electrolyte in the  samples, four exothermic peaks were detected. First, the metastable compounds in SEI film break down when the temperature reaches
samples, four exothermic peaks were detected. First, the metastable compounds in SEI film break down when the temperature reaches  (mean value), and this exotherm peaks at about
(mean value), and this exotherm peaks at about  . Then, the electrolyte can penetrate the broken SEI film and reach the edge planes of the lithiated graphite, and reacts with the lithium diffusing from the inner structure; hence, a secondary SEI film is formed. This reaction does not stop until either the intercalated lithium is consumed completely or products such as
. Then, the electrolyte can penetrate the broken SEI film and reach the edge planes of the lithiated graphite, and reacts with the lithium diffusing from the inner structure; hence, a secondary SEI film is formed. This reaction does not stop until either the intercalated lithium is consumed completely or products such as  and LiF completely block the surface of edge planes. The secondary SEI decomposes with temperature rising; then, the remaining intercalated lithium in the graphite structure will be released from the graphite structure. The lithium released from graphite particle reacts with PVDF and electrolyte decomposition by-products to generate more heat.
and LiF completely block the surface of edge planes. The secondary SEI decomposes with temperature rising; then, the remaining intercalated lithium in the graphite structure will be released from the graphite structure. The lithium released from graphite particle reacts with PVDF and electrolyte decomposition by-products to generate more heat.
Acknowledgments
This study was supported by "100 Talents Project" of Chinese Academy of Sciences and China NKBRSF project, no. 2001CB409600 . Financial support from Nature Science Funds of Anhui Province, no. 050450403 , is also appreciated.
University of Science and Technology of China assisted in meeting the publication costs of this article.