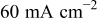Abstract
Localized hydrogen evolution was found in the anode flow field of a liquid-feed direct methanol fuel cell (DMFC) under open-circuit conditions after the oxygen supply was cut off or restarted. More importantly, this phenomenon led to a temporary rise in the performance of the DMFC by as much as 7%. The relationship among oxygen supply interruption, hydrogen evolution, and performance variation has been investigated both theoretically and experimentally.
Export citation and abstract BibTeX RIS
Because direct methanol fuel cells (DMFCs) are considered as one of the most competitive candidates for the future power sources of transportation and portable applications, they have recently attracted considerable attention.1–8 Presently, however, there still exist two major challenges that must be overcome before the widespread commercialization of this type of fuel cell is possible. The first is that the electrochemical oxidation of methanol is sluggish; hence, highly reactive anodic catalysts are needed. The second, more serious, problem is methanol crossover through the Nafion type membranes to the cathodic side.9–11 It is well known that methanol crossover not only causes a large overpotential on the cathode but also reduces the fuel utilization, especially under open-circuit conditions (OCC). In addition, the permeated methanol is electrochemically oxidized by oxygen on the cathode at typical cathode catalyst sites,12 which makes both thermal and water management in DMFCs more complex. In spite of the significant improvements in DMFC performance, especially in the past 15 years,2, 11 a better understanding of both electrochemical and mechanical characteristics in a DMFC running under different conditions is essential for system optimization and reliable operation of the fuel cell. Gas evolution on the anode of a DMFC under OCC might have been noticed by some researchers, but this interesting phenomenon has been neglected or simply explained as an occasional behavior caused by evaporation of methanol or leakage of oxygen. In this work, we repeatedly found that gas bubbles appeared in the anode flow field shortly after the DMFC system was shut down. A similar phenomenon was also found after the DMFC was restarted. In our laboratory, we have repeatedly found that every time the DMFC was restarted, the cell voltages over the entire range of current densities became significantly higher than those collected before the system shut down. This performance improvement, however, is temporary in nature; the cell performance degraded to a steady state when the DMFC was operated for a long enough period of time. This study aims to reveal the relationship among oxygen interruption, hydrogen evolution, and performance variation.
Experimental
Membrane-electrode assembly (MEA)
An MEA with an active area of  was fabricated in-house employing two single-side ELAT electrodes from E-TEK and a Nafion 115 membrane. Both anode and cathode electrodes used carbon cloth (E-TEK, Type A) as the backing support layer with 30% PTFE wet-proofing treatment. The catalyst loading on the anode side was
was fabricated in-house employing two single-side ELAT electrodes from E-TEK and a Nafion 115 membrane. Both anode and cathode electrodes used carbon cloth (E-TEK, Type A) as the backing support layer with 30% PTFE wet-proofing treatment. The catalyst loading on the anode side was  with unsupported [Pt:Ru] Ox (1:1 atom %), while the catalyst loading on the cathode side was
with unsupported [Pt:Ru] Ox (1:1 atom %), while the catalyst loading on the cathode side was  using 40% Pt on Vulcan XC-72. The final MEA was formed by hot pressing at 135°C and 5 MPa for 3 min.
using 40% Pt on Vulcan XC-72. The final MEA was formed by hot pressing at 135°C and 5 MPa for 3 min.
Single cell assembly and flow visualization
The MEA was inserted into an in-house fabricated single cell fixture. To visualize two-phase transport phenomena inside flow field channels without sacrificing accuracy and convenience of temperature control, the cell fixture was made of transparent poly(methyl methacrylate) (PMMA) for one side and aluminum for the other. In this study, the transparent PMMA fixture served as the anode side for identification of gas evolution. Both current collector plates were made of stainless steel 316L machined by wire-cut technology. In this study, a single serpentine channel, having 1.3 mm channel width, 1.0 mm rib width, and 1.0 mm depth, was formed on both the anode and cathode sides.
Cell test station and test conditions
Experiments were carried out in a DMFC test station detailed elsewhere.13 Briefly, 2 M methanol solution was pumped to the cell by a digital HPLC micro-pump (Series III) and the flow rate was fixed at  . Dry pure oxygen was fed to the cathode of the cell and the flow rate was fixed at 100 standard cubic centimeters per minute (sccm) and controlled by a mass flow controller (Omega FMA-7105E). The operating temperature of the cell as well as the temperature of the methanol and oxygen feed were maintained at 60°C. The performance data reported in this paper were collected after a 3 day MEA activa tion period.
. Dry pure oxygen was fed to the cathode of the cell and the flow rate was fixed at 100 standard cubic centimeters per minute (sccm) and controlled by a mass flow controller (Omega FMA-7105E). The operating temperature of the cell as well as the temperature of the methanol and oxygen feed were maintained at 60°C. The performance data reported in this paper were collected after a 3 day MEA activa tion period.
Results and Discussion
As in previous studies,13 carbon dioxide gas bubbles were observed in the anode flow field channels as the DMFC was discharged at a sufficiently large current density. On the other hand, no gas bubbles were observed in the flow field after the external load was completely removed (the open-circuit condition) while keeping both the methanol solution and oxygen supply open. Under the open-circuit condition, however, gas bubbles appeared in the anode flow field shortly after the oxygen supply was cut off while keeping the methanol solution feed open. Typical gas bubble behavior in the flow field is shown in Fig. 1. We found that every time the oxygen feed supply was cut off, the gas bubble evolution repeatedly appeared, lasted 2-4 min, and ceased eventually. Interestingly, the gas bubble evolution restarted immediately after the oxygen supply was restarted, lasting several seconds only. Chromatographic analysis showed that the gas evolved in the flow field principally consisted of  and
and  with small amounts of methanol and water vapor.
with small amounts of methanol and water vapor.
Figure 1. Gas evolution on the anode shortly after the DMFC was shut down.
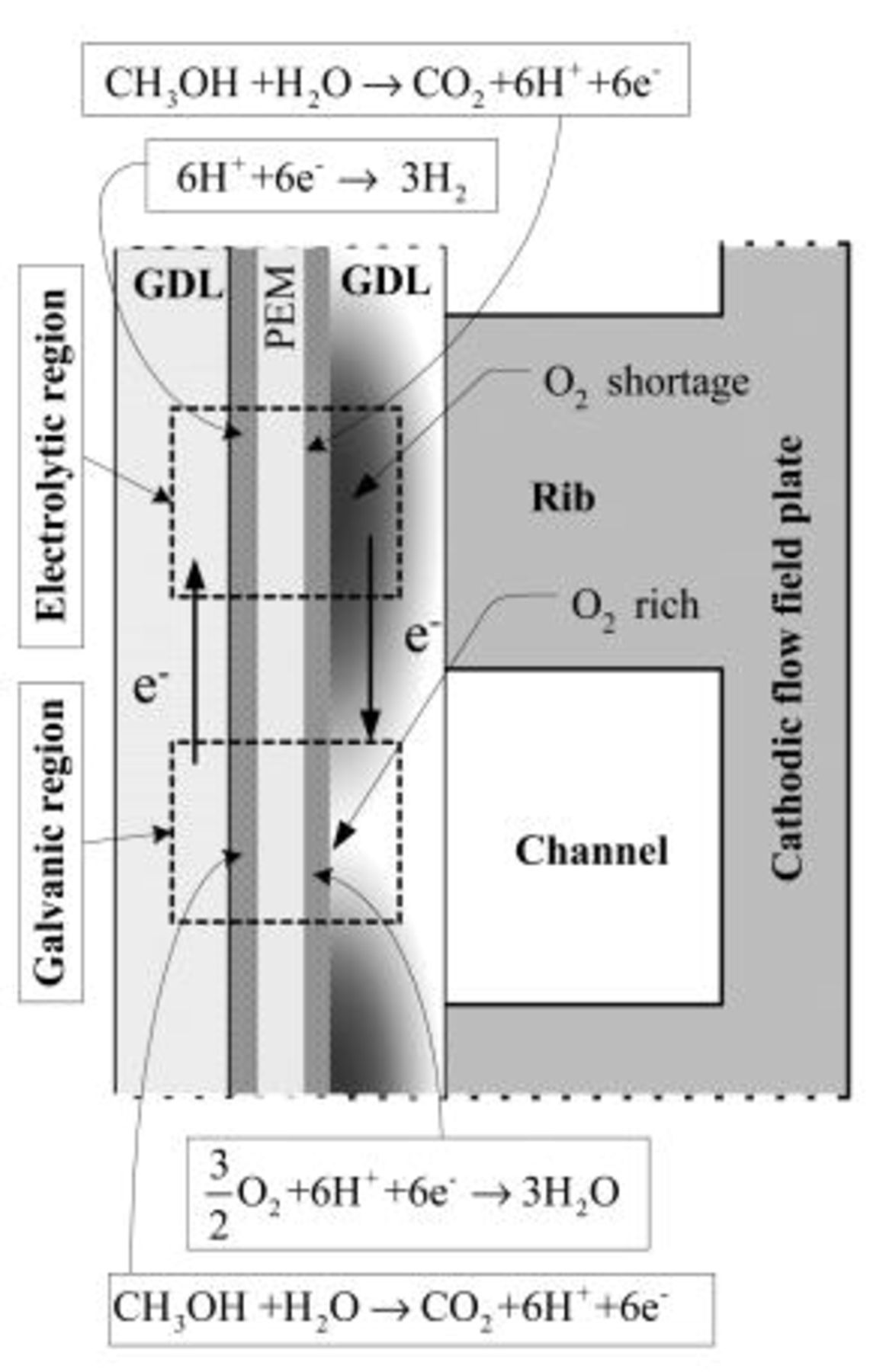
The mechanism leading to the gas evolution in the anode flow field under the conditions after the external electrical load is removed and the oxygen supply is cut off or started up can be explained as follows. Because the methanol solution feed is kept open, the crossover of methanol and water from the anode to the cathode continues. Thus, on the cathode, the permeated methanol reacts with oxygen, if available. After the oxygen supply is cut off, the reaction between the permeated methanol and oxygen is sustained primarily by the residual oxygen in the flow field channels. As schematically illustrated in Fig. 2, this reaction will cause oxygen in the region under the channel ribs to be exhausted first, forming an oxygen depletion region. On the other hand, in the region under the channel openings oxygen still remains relatively richer, forming a localized oxygen-rich region. Although the two regions are connected in parallel by both of the electrodes and the same electrolyte (membrane), the lateral proton conduction in the electrolyte is so low that the oxygen-lean and oxygen-rich sections of the cell act as if they were two independent cells that are connected electrically but not ionically.14 The oxygen depletion region acts as an electrolytic cell, whereas the oxygen rich region still acts as a galvanic cell (the normal DMFC operation) that provides a voltage between the two electrodes. In the oxygen depletion region, the following electrolytic reactions take place
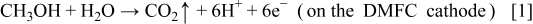
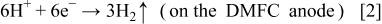
The above reactions are powered by the following galvanic cell reactions
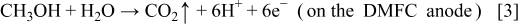
and
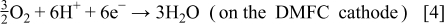
which take place in the oxygen rich-region. Reactions 2 and 3 explain why the gas evolution was observed in the anode flow field of the DMFC under open-circuit conditions after the oxygen feed was cut off. It should be pointed out that because most of  dissolved in methanol solution, the visible gas bubbles mainly consisted of hydrogen. Reactions 1-4 originate from the nonuniform distribution of oxygen on the cathode caused by the oxygen supply cutoff. Therefore, hydrogen evolution, Reaction 2, lasting several minutes, will cease when the residual oxygen in the cell is consumed. In a fashion similar to the oxygen supply cutoff, the oxygen supply startup also causes a non-uniform distribution of oxygen on the cathode, thereby leading to hydrogen evolution, Reaction 2, which lasts several seconds and ceases when the oxygen distribution in the cathode flow field becomes more uniform under a steady state. Hence, the hydrogen evolution, Reaction 2, induced by the oxygen supply interruption (either cutoff or startup) is a behavior that is transient in nature. In our previous work,14 a constant hydrogen evolution process was found under open-circuit conditions when the oxygen feed flow rate was kept to be sufficiently low. Nevertheless, both the transient and constant hydrogen evolutions are induced by the nonuniform distribution of oxygen on the cathode.
dissolved in methanol solution, the visible gas bubbles mainly consisted of hydrogen. Reactions 1-4 originate from the nonuniform distribution of oxygen on the cathode caused by the oxygen supply cutoff. Therefore, hydrogen evolution, Reaction 2, lasting several minutes, will cease when the residual oxygen in the cell is consumed. In a fashion similar to the oxygen supply cutoff, the oxygen supply startup also causes a non-uniform distribution of oxygen on the cathode, thereby leading to hydrogen evolution, Reaction 2, which lasts several seconds and ceases when the oxygen distribution in the cathode flow field becomes more uniform under a steady state. Hence, the hydrogen evolution, Reaction 2, induced by the oxygen supply interruption (either cutoff or startup) is a behavior that is transient in nature. In our previous work,14 a constant hydrogen evolution process was found under open-circuit conditions when the oxygen feed flow rate was kept to be sufficiently low. Nevertheless, both the transient and constant hydrogen evolutions are induced by the nonuniform distribution of oxygen on the cathode.
Figure 2. Electrochemical reactions under open-circuit conditions induced by  interruption.
interruption.
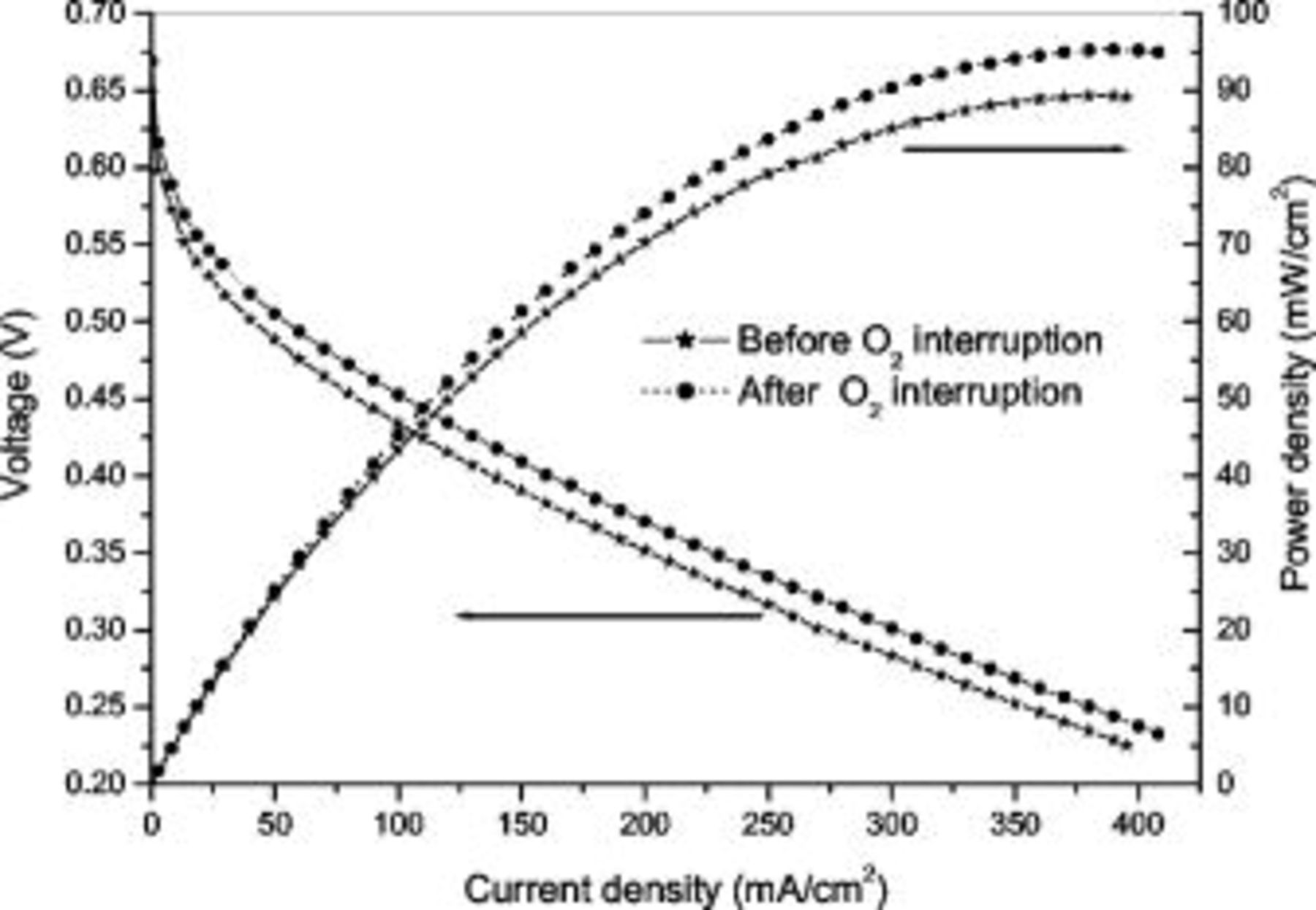
In addition to the gas evolution discussed above, we also found that the oxygen supply interruption (cutoff followed by restart-up) caused a temporary rise in the cell performance. As shown in Fig. 3, the cell voltages over the entire range of current densities measured after a 5 min oxygen interruption (represented by dot symbols) were significantly higher than those measured before the oxygen interruption break (represented by star symbols). We further found that the cell performance returned to the level that existed before the oxygen interruption after the cell was operated continuously for a sufficient period of time. This change in the cell performance was observed under OCC whenever the oxygen supply was cut off and restarted. To show this periodic variation in the cell performance caused by oxygen interruptions, we tested the cell by following a testing cycle: 5 min open circuit condition (OCC) operation, 40 min staircase discharge operation, and 10 min discharge at  . The variation in the measured voltages for two selected current densities (50 and
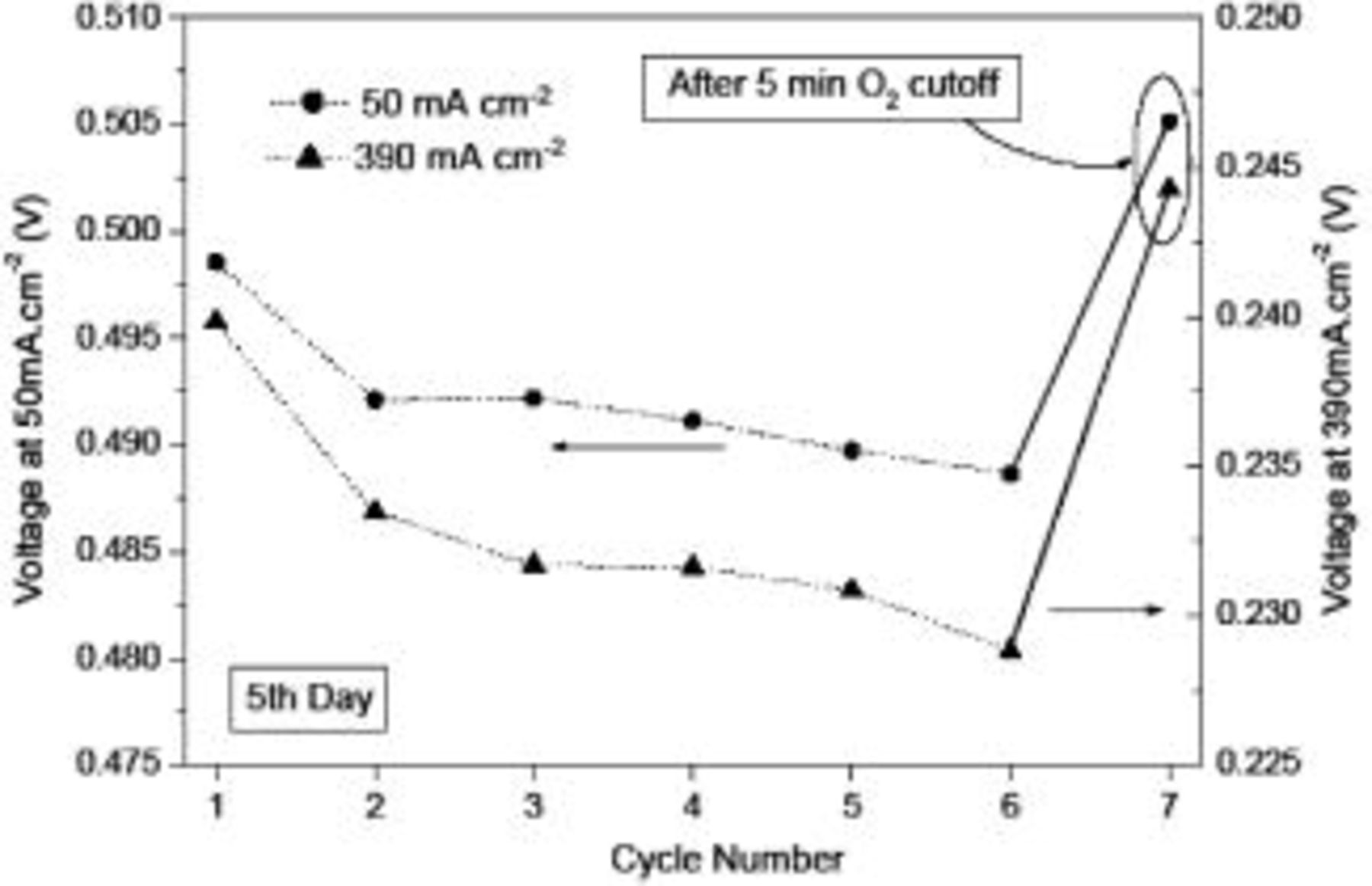
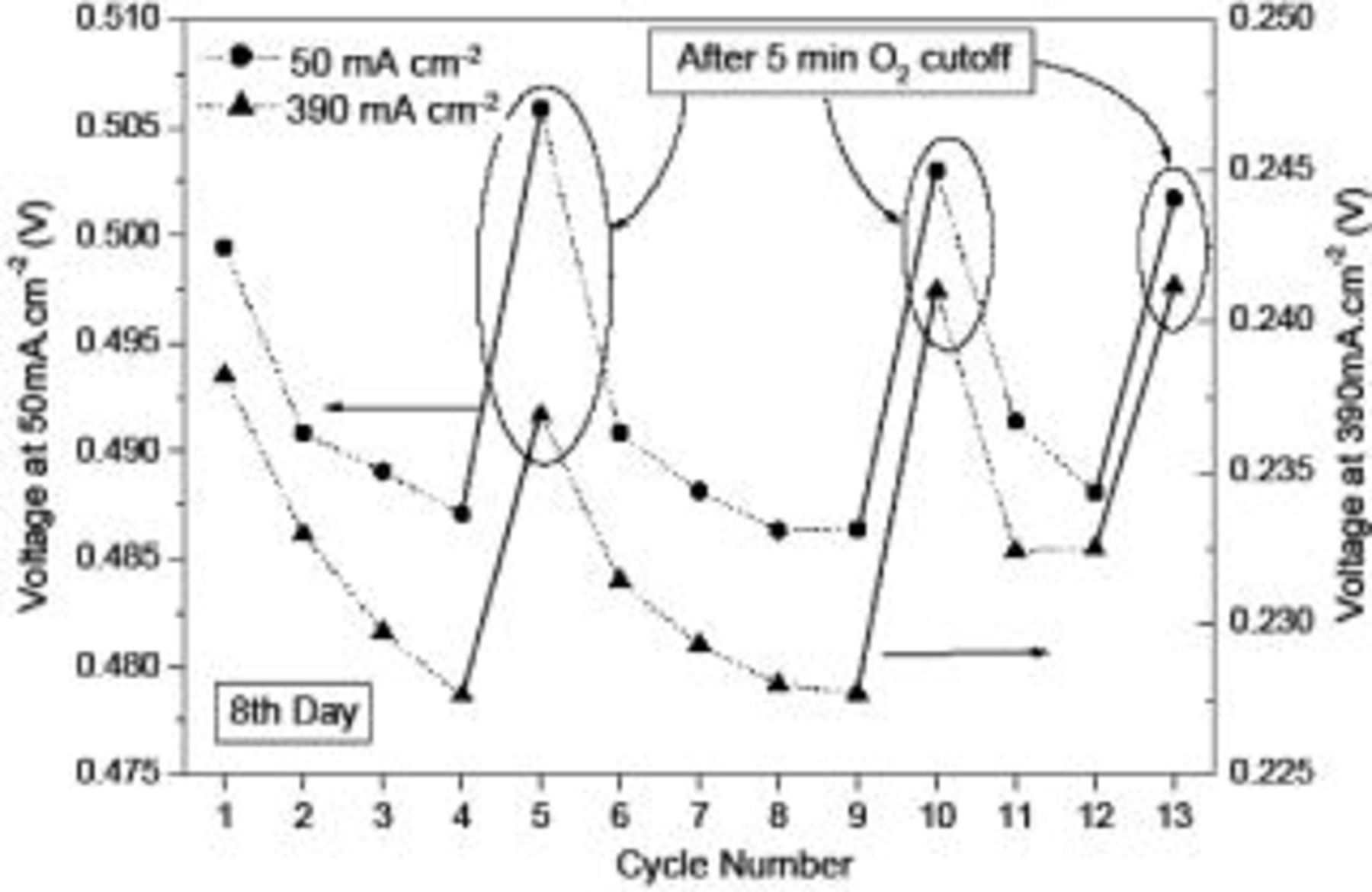
. The variation in the measured voltages for two selected current densities (50 and  ) over seven consecutive testing cycles performed on the fifth day (the age of the MEA) is shown in Fig. 4. Note that there were no oxygen interruptions from the first to the sixth cycle, but the oxygen supply was cut off after the sixth cycle and restarted 5 minutes later (before the seventh cycle). It is seen from Fig. 4 that the voltages exhibited a more rapid decrease in the first three cycles and the decrease became slower toward the sixth cycle. After the 5 min oxygen interruption between the sixth and seventh cycle, however, the voltages increased significantly. To confirm this interesting phenomenon, we repeated the above experiments for 13 consecutive cycles with three oxygen interruption operations on the eighth day. The results are shown in Fig. 5. Apparently, the voltage corresponding to each selected current density exhibited a significant increase from the fourth to fifth cycle, from the ninth to tenth cycle, and from the twelfth to thirteenth cycle whenever the oxygen supply was closed for 5 min. On the other hand, the voltage decreased gradually between two consecutive cycles without oxygen interruptions. It was found that the maximum difference in the voltages measured on the same day was as high as about 7% at
) over seven consecutive testing cycles performed on the fifth day (the age of the MEA) is shown in Fig. 4. Note that there were no oxygen interruptions from the first to the sixth cycle, but the oxygen supply was cut off after the sixth cycle and restarted 5 minutes later (before the seventh cycle). It is seen from Fig. 4 that the voltages exhibited a more rapid decrease in the first three cycles and the decrease became slower toward the sixth cycle. After the 5 min oxygen interruption between the sixth and seventh cycle, however, the voltages increased significantly. To confirm this interesting phenomenon, we repeated the above experiments for 13 consecutive cycles with three oxygen interruption operations on the eighth day. The results are shown in Fig. 5. Apparently, the voltage corresponding to each selected current density exhibited a significant increase from the fourth to fifth cycle, from the ninth to tenth cycle, and from the twelfth to thirteenth cycle whenever the oxygen supply was closed for 5 min. On the other hand, the voltage decreased gradually between two consecutive cycles without oxygen interruptions. It was found that the maximum difference in the voltages measured on the same day was as high as about 7% at  and more than 3% at
and more than 3% at  .
.
Figure 3. Cell performance comparison before and after  interruption.
interruption.
Figure 4. Measured voltages corresponding to 50 and  over 7 cycles with
over 7 cycles with  interrupted after the sixth cycle.
interrupted after the sixth cycle.
Figure 5. Measured voltages corresponding to  and
and  over 13 cycles with
over 13 cycles with  interrupted after the fourth, ninth, and twelfth cycle.
interrupted after the fourth, ninth, and twelfth cycle.
The above discussion indicates that oxygen supply interruptions led to electrolytic hydrogen evolution, Reaction 2, which was accompanied by a significant increase in the cell performance. This coincidence leads us to believe that the increase in the cell performance has something to do with electrolytic hydrogen evolution. Interestingly, He et al.15 found that hydrogen evolution on the electrodes, induced deliberately by applying an external power source, is an effective way to improve performance of an air cathode and methanol anode. They concluded that the mechanism leading to such an improvement in cell performance was due to an increase in catalyst utilization, which results from the change in porosity and tortuosity in the catalyst layers by electrolytic hydrogen evolution. In this work, we also investigated the effect of electrolytic hydrogen evolution induced by an external power source on the cell performance. The electrolytic reaction on the anode of the DMFC was induced using a potentiostat (EG&G 273A) between testing cycles. Detailed procedures are described as follows:
- (1)Purging the cathode with nitrogen. This was undertaken under the discharging condition to avoid the internal hydrogen evolution caused by the nonuniform distribution of oxygen under OCC.
- (2)Applying a current density of
 to the cell (by maintaining cell voltage at 0.5 V) for 5 min using the potentiostat with the working electrode and counter electrode (together with reference electrode) connected to the cell cathode and anode, respectively.
to the cell (by maintaining cell voltage at 0.5 V) for 5 min using the potentiostat with the working electrode and counter electrode (together with reference electrode) connected to the cell cathode and anode, respectively. - (3)Switching back to oxygen cathode feed from nitrogen under the discharging condition.
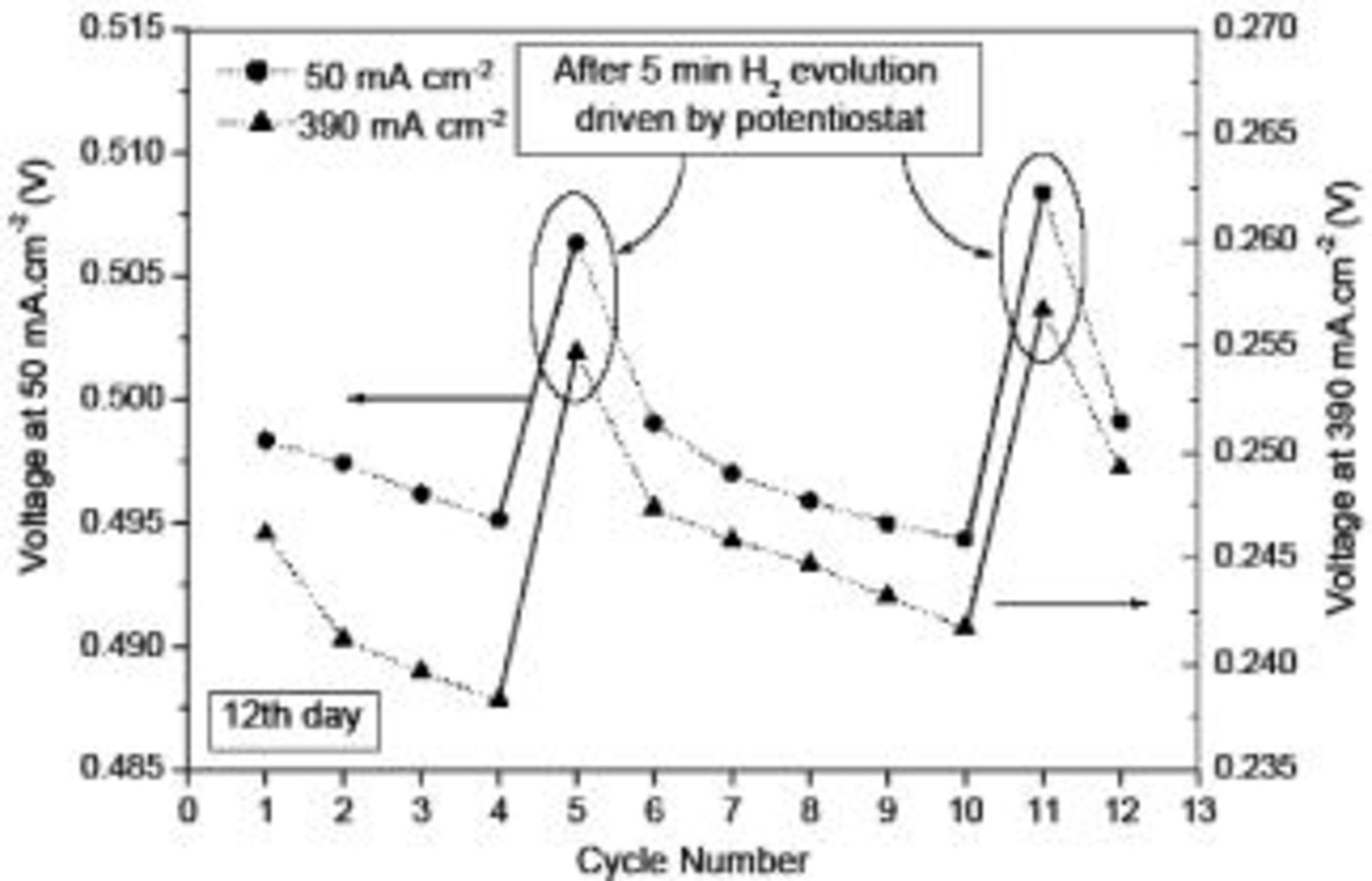
After the first and second steps, the electrolytic reactions, described in Reactions 1 and 2, took place on the cathode and the anode, respectively. We performed the experiments for 12 consecutive cycles on the twelfth day with the external hydrogen evolution induced between some cycles. The variation in the measured voltage at 50 and  is presented in Fig. 6. As can be seen from Fig. 6, electrolytic hydrogen evolution induced by the external power source between the fourth and fifth cycle and between the tenth and eleventh cycle also caused a significant increase in the cell voltage; and the voltage decreased gradually between the cycles without electrolytic hydrogen evolution. Note that this external electrolytic hydrogen evolution also led to an increase in the cell voltage by about 7% at
is presented in Fig. 6. As can be seen from Fig. 6, electrolytic hydrogen evolution induced by the external power source between the fourth and fifth cycle and between the tenth and eleventh cycle also caused a significant increase in the cell voltage; and the voltage decreased gradually between the cycles without electrolytic hydrogen evolution. Note that this external electrolytic hydrogen evolution also led to an increase in the cell voltage by about 7% at  , implying that the effect of both the external and internal electrolytic hydrogen evolution on the cell performance was almost the same.
, implying that the effect of both the external and internal electrolytic hydrogen evolution on the cell performance was almost the same.
Figure 6. Measured voltages corresponding to  and
and  over 12 cycles with
over 12 cycles with  evolution induced by an external power source after the fourth and tenth cycle.
evolution induced by an external power source after the fourth and tenth cycle.
The results presented above suggest that no matter how the hydrogen evolution reaction is induced, by oxygen interruption or by using an external power source, it indeed affects the cell performance. Although the hydrogen evolution induced by oxygen interruption is a localized (under the cathode ribs) and temporary (lasting several minutes at most) behavior in nature, it has almost the same effect on the cell performance as the hydrogen evolution reaction induced by an external power source, which occurs continuously in the entire MEA surface. Note that for the present fully activated MEA, hydrogen evolution led to a temporary cell improvement only; the cell performance gradually degraded to a stable value when the cell discharged continuously for a sufficiently long period without oxygen interruptions. Note also that the reaction rates of the galvanic and electrolytic reactions given by Reaction 1-4 depend on temperature and the methanol permeation rate. The phenomena of hydrogen evolution and the induced performance variation are expected to become more pronounced for the DMFCs with a thinner electrolyte membrane running at higher temperatures and with higher methanol concentrations. Although the hydrogen evolution reaction has been found to affect electrode performance both in this work and in a previous study,15 and a preliminary explanation for this effect has been given,15 a complete understanding of the underlying mechanism leading to this significant phenomenon at the catalyst-electrolyte-reactant interfaces is an important future research topic.
Conclusions
Localized hydrogen evolution has been observed in the anode flow field of a DMFC under open-circuit conditions after the oxygen supply was cutoff or restarted. This unique phenomenon in DMFCs has been attributed to the problem of methanol crossover from the anode to the cathode and is induced by the local shortage of oxygen on the cathode. The self-sustained electrolytic reaction has been found to cause a temporary rise in the performance of the DMFC. This finding has been verified by the electrolytic hydrogen evolution generated by an external power source. The shutdown and startup of a DMFC system are inevitable not only in laboratory investigations but also in practical applications. There exists a significant difference in the performance data collected before and after the system shutdown. A valid comparison can be made only when a DMFC is operated continuously for a sufficiently long time without oxygen interruptions. In addition, for a DMFC stack with a large MEA area, caution may need to be taken for the hydrogen accumulation caused by the system shutdown and startup.
Acknowledgments
The work described in this paper was fully supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region, China (project no. HKUST6193/01E).
The Hong Kong University of Science and Technology assisted in meeting the publication costs of this article.