Abstract
Small amounts of metal ions  or
or  added to an electrolyte for Mn electrodeposition increase the metallic character of amorphous Mn coatings grown at high current densities. The codeposited oxygen decreases from 54 atom % to less than 7 atom %, while the codeposited Sn or Cu are below 1 and 3 atom %, respectively. The resulting amorphous coatings exhibit very different mechanical properties and better corrosion resistance than those of amorphous manganese coatings electrodeposited without metal ion additives. © 2004 The Electrochemical Society. All rights reserved.
added to an electrolyte for Mn electrodeposition increase the metallic character of amorphous Mn coatings grown at high current densities. The codeposited oxygen decreases from 54 atom % to less than 7 atom %, while the codeposited Sn or Cu are below 1 and 3 atom %, respectively. The resulting amorphous coatings exhibit very different mechanical properties and better corrosion resistance than those of amorphous manganese coatings electrodeposited without metal ion additives. © 2004 The Electrochemical Society. All rights reserved.
Export citation and abstract BibTeX RIS
The introduction of small amounts of additives to a solution for aqueous electrodeposition of a metal can induce relevant changes in the nature and properties of the films grown at the cathode. These changes may include brightening of the deposit, leveling, as well as variations in the mechanical and physical properties, reduction in the internal stresses, and improvement in the corrosion resistance of the coatings.1
2
3 Metal ions in particular may be added to act as grain refiners. While studying the effect of small additions of  or
or  on the Mn electroplating process, we found that small additions of metal ions may also have a profound influence on the chemical state of the deposit and consequently on its mechanical and corrosion resistance properties.
on the Mn electroplating process, we found that small additions of metal ions may also have a profound influence on the chemical state of the deposit and consequently on its mechanical and corrosion resistance properties.
Electrodeposited coatings of manganese and its alloys have been studied as a replacement for cadmium in the sacrificial protection of steel, due to their environmentally friendly nature, potentially high corrosion protection performance, good tribological behavior, and suitable mechanical properties.3
4
5
6
7
8 Pure manganese has not been formerly considered as a protective coating for steel because of its high chemical reactivity and brittle nature.8
9
10
11 Therefore, alloying of Mn with other more noble metals, such as Sn,12 Zn9
13
14
15
16
17
18 or iron group elements,19
20
21
22
23 has been attempted to reduce its reactivity. The brittle nature of electrodeposited Mn originates from the room-temperature phase transformation of as-deposited ductile γ-Mn (centered tetragonal, ct), to the brittle α form (body-centered cubic, bcc). It has been shown that this transformation may be prevented effectively by codepositing Mn with face-centered cubic (fcc) metals, such as Cu.8 In this work, the effect of the addition of small amounts of  or
or  to Mn electroplating solutions on the resulting coatings was studied. The structural, chemical, mechanical, and corrosion properties of these coatings were investigated. Significant differences in the chemical state of the resulting coatings as well as on their properties were induced by the addition of
to Mn electroplating solutions on the resulting coatings was studied. The structural, chemical, mechanical, and corrosion properties of these coatings were investigated. Significant differences in the chemical state of the resulting coatings as well as on their properties were induced by the addition of  or
or  ions.
ions.
Experimental
Electrochemical and electrodeposition setups are described in detail in Ref. 11. The solutions contained 0.59 M manganese sulfate  and 1 M ammonium sulfate
and 1 M ammonium sulfate  with the addition of 0.001-0.01 M stannous sulfate
with the addition of 0.001-0.01 M stannous sulfate  or 0.0025-0.02 M cupric sulfate
or 0.0025-0.02 M cupric sulfate  pH was adjusted to 2.6-2.8 by adding sulfuric acid. The Sn-Mn solutions were stable only up to these pH values, and Cu-Mn solutions were well buffered and stable only in this pH range and at pH 6.6-6.8. All solutions were freshly prepared with analytical grade reagents (Fisher Scientific) and triply distilled water. Experiments were carried out at 25°C and under quiescent conditions.
pH was adjusted to 2.6-2.8 by adding sulfuric acid. The Sn-Mn solutions were stable only up to these pH values, and Cu-Mn solutions were well buffered and stable only in this pH range and at pH 6.6-6.8. All solutions were freshly prepared with analytical grade reagents (Fisher Scientific) and triply distilled water. Experiments were carried out at 25°C and under quiescent conditions.
Surface morphology of the coatings was examined by scanning electron microscopy (SEM) using a Philips XL30 instrument. Chemical analysis of the deposits was performed by an attached energy-dispersive X-ray spectrometer (EDX) with a CDU LEAP detector. Incorporation of oxygen and the chemical states of each element were further studied by X-ray photoelectron spectroscopy (XPS), using a Kratos Axis 165 system with a monochromatic Al Kα  source (for Mn and Sn-Mn coatings) and Mg Kα
source (for Mn and Sn-Mn coatings) and Mg Kα  source (for Cu-Mn) at a pass energy of 160 eV (survey scans) or 40 eV (high-resolution scans). The use of Mg Kα radiation allowed us to distinguish between the Mn
source (for Cu-Mn) at a pass energy of 160 eV (survey scans) or 40 eV (high-resolution scans). The use of Mg Kα radiation allowed us to distinguish between the Mn  and Cu
and Cu  characteristic transitions,24 as well as between the Mn
characteristic transitions,24 as well as between the Mn  Auger peak and the shake-up peak of Cu(II)
Auger peak and the shake-up peak of Cu(II)  The Mn 2p peak was analyzed with the charge neutralizer on to avoid surface charging effects.25
26 The atomic fraction of each element was evaluated semiquantitatively by weighing the corresponding signals after subtracting a Shirley-type background.25 Binding energies were calibrated using the adventitious C 1s peak at 285.0 eV as a reference. Crystal structure was determined by X-ray diffraction (XRD), using θ-2θ geometry with a Rigaku D/Max-2BX XRD system with Cu Kα radiation. A Nano Indenter II mechanical properties microprobe (Nano Instruments, Oak Ridge, TN) equipped with a nanoscratch attachment was used to perform the constant load tribological experiments, using a Berkovich diamond tip. The nanoindentation hardness and Young's modulus were also measured using a Hysitron Triboscope nanomechanical test instrument. Corrosion resistance and possible passivation behavior of the coatings were measured by anodic polarization in 3% NaCl solution at pH 3.0. The experimentally determined polarization curves were fitted using the Stern-Geary equation to give the values of the corrosion potential
The Mn 2p peak was analyzed with the charge neutralizer on to avoid surface charging effects.25
26 The atomic fraction of each element was evaluated semiquantitatively by weighing the corresponding signals after subtracting a Shirley-type background.25 Binding energies were calibrated using the adventitious C 1s peak at 285.0 eV as a reference. Crystal structure was determined by X-ray diffraction (XRD), using θ-2θ geometry with a Rigaku D/Max-2BX XRD system with Cu Kα radiation. A Nano Indenter II mechanical properties microprobe (Nano Instruments, Oak Ridge, TN) equipped with a nanoscratch attachment was used to perform the constant load tribological experiments, using a Berkovich diamond tip. The nanoindentation hardness and Young's modulus were also measured using a Hysitron Triboscope nanomechanical test instrument. Corrosion resistance and possible passivation behavior of the coatings were measured by anodic polarization in 3% NaCl solution at pH 3.0. The experimentally determined polarization curves were fitted using the Stern-Geary equation to give the values of the corrosion potential  and the corrosion current
and the corrosion current  using proprietary software.
using proprietary software.
Results and Discussion
Morphological and structural characteristics.—
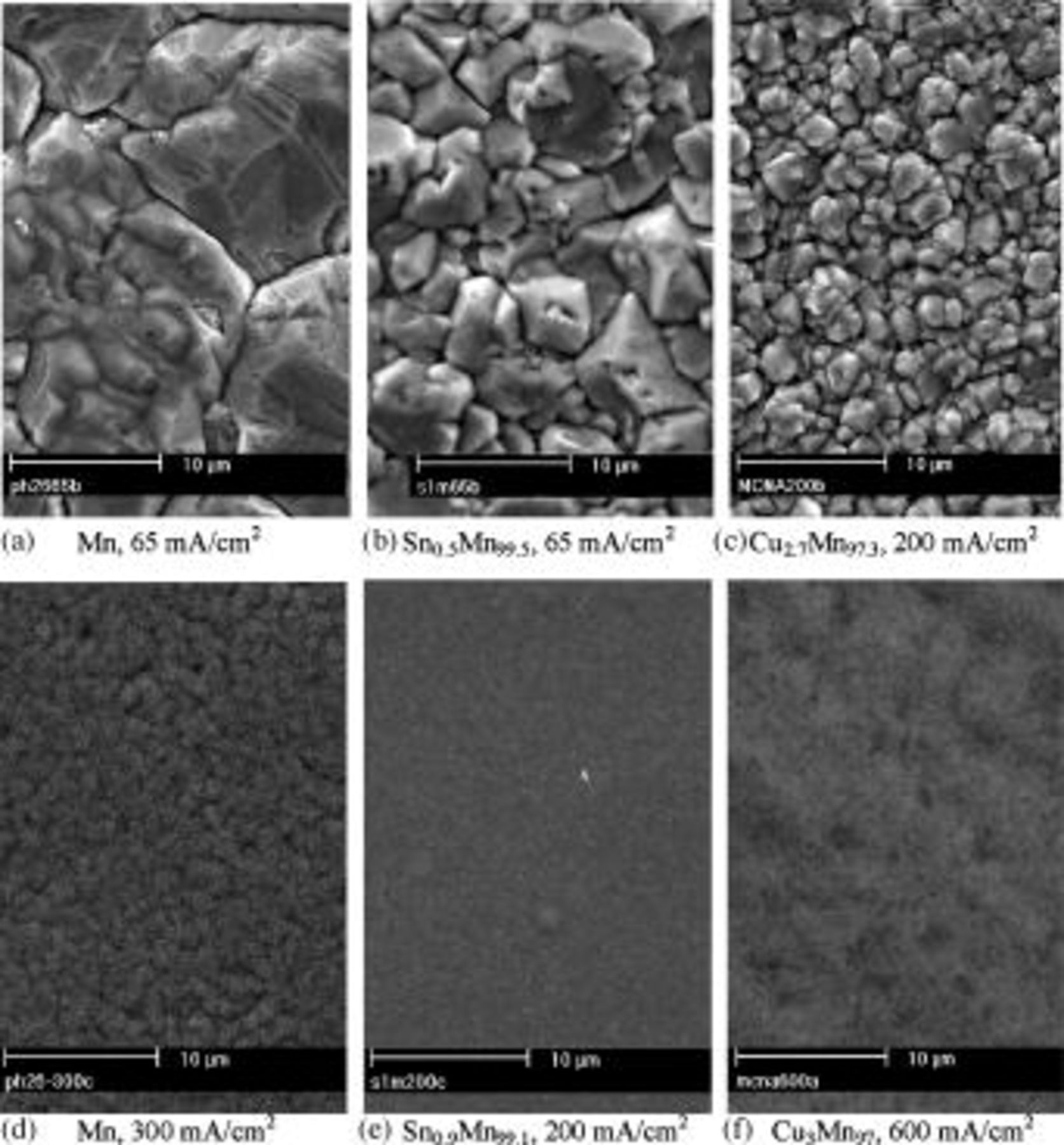
Galvanostatic electrodeposition of Mn,11 Sn-Mn,12 and Cu-Mn yielded two types of sound coatings, crystalline (type I) or amorphous (type II), in dependence of the applied current density. At high current density, amorphous coatings were obtained in all cases. Under these electrodeposition conditions, hydrogen evolution was very strong and consequently stirring did not change the limiting current. The content of Sn in amorphous Sn-Mn coatings, and of Cu in amorphous Cu-Mn coatings, was less than 1 and 3 atom %, respectively, almost independent of current density. The conditions for the growth of amorphous Cu-Mn coatings at pH 6.6-6.8 were similar to those at pH 2.6-2.8, therefore we assumed that the effect of pH on the formation of amorphous coatings was minimal. The morphology, composition (by EDX, considering only the metallic elements), and growth parameters of typical coatings are shown in Fig. 1. The grain size of type I Mn coatings systematically decreased upon codeposition of small amounts of Sn or Cu. A higher concentration of  or
or  tended to increase the minimum current density necessary to obtain type II coatings.
tended to increase the minimum current density necessary to obtain type II coatings.
Figure 1. SEM secondary electron surface micrographs of type I and II coatings electrodeposited at various current densities at pH 2.6-2.8. The electrolytes contain  (0.59 M),
(0.59 M),  (1 M) and (b) and (e)
(1 M) and (b) and (e)  (0.001 M); (c) and (f)
(0.001 M); (c) and (f)  (0.005 M).
(0.005 M).
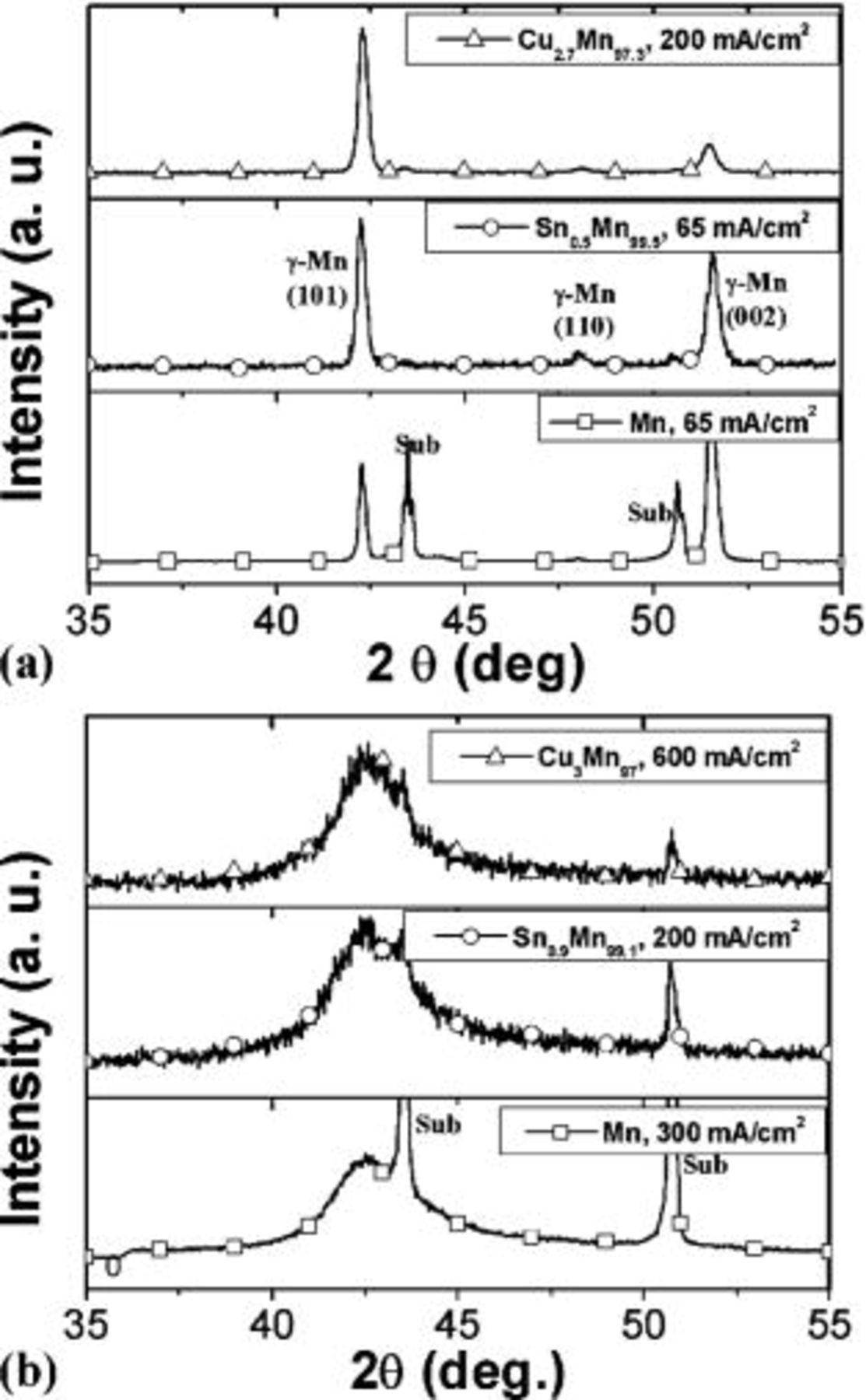
XRD patterns of these films are compared in Fig. 2. As-deposited type I coatings all exhibit the γ(ct) structure, a metastable solid solution in the bulk alloy. No solubility of Cu and little solubility of Sn in Mn at room temperature has been reported in the corresponding equilibrium phase diagrams.27 No separate peaks of Sn or Cu phases were discerned in our experiments. Both Mn and Sn-Mn crystalline coatings underwent phase transformation from γ to the α-phase (bcc) in several weeks at room temperature. A small amount of Cu (3-4 atom %) on the contrary can stabilize the as-deposited γ form for over 18 months at room temperature. All type II amorphous coatings exhibited a stable amorphous structure. No crystallization was detected by XRD for these films after over 18 months aging at room temperature.
Figure 2. XRD patterns of as-deposited type (a) I and (b) II coatings obtained at pH 2.6-2.8.
Chemical states.—
With the addition of  and
and  in the electrolytes, the appearance of the amorphous coatings was bright and glossy, in contrast with that of type II pure Mn coatings, which is black and glossy.11 XPS was used to obtain detailed information about the chemical composition of type II coatings.
in the electrolytes, the appearance of the amorphous coatings was bright and glossy, in contrast with that of type II pure Mn coatings, which is black and glossy.11 XPS was used to obtain detailed information about the chemical composition of type II coatings.
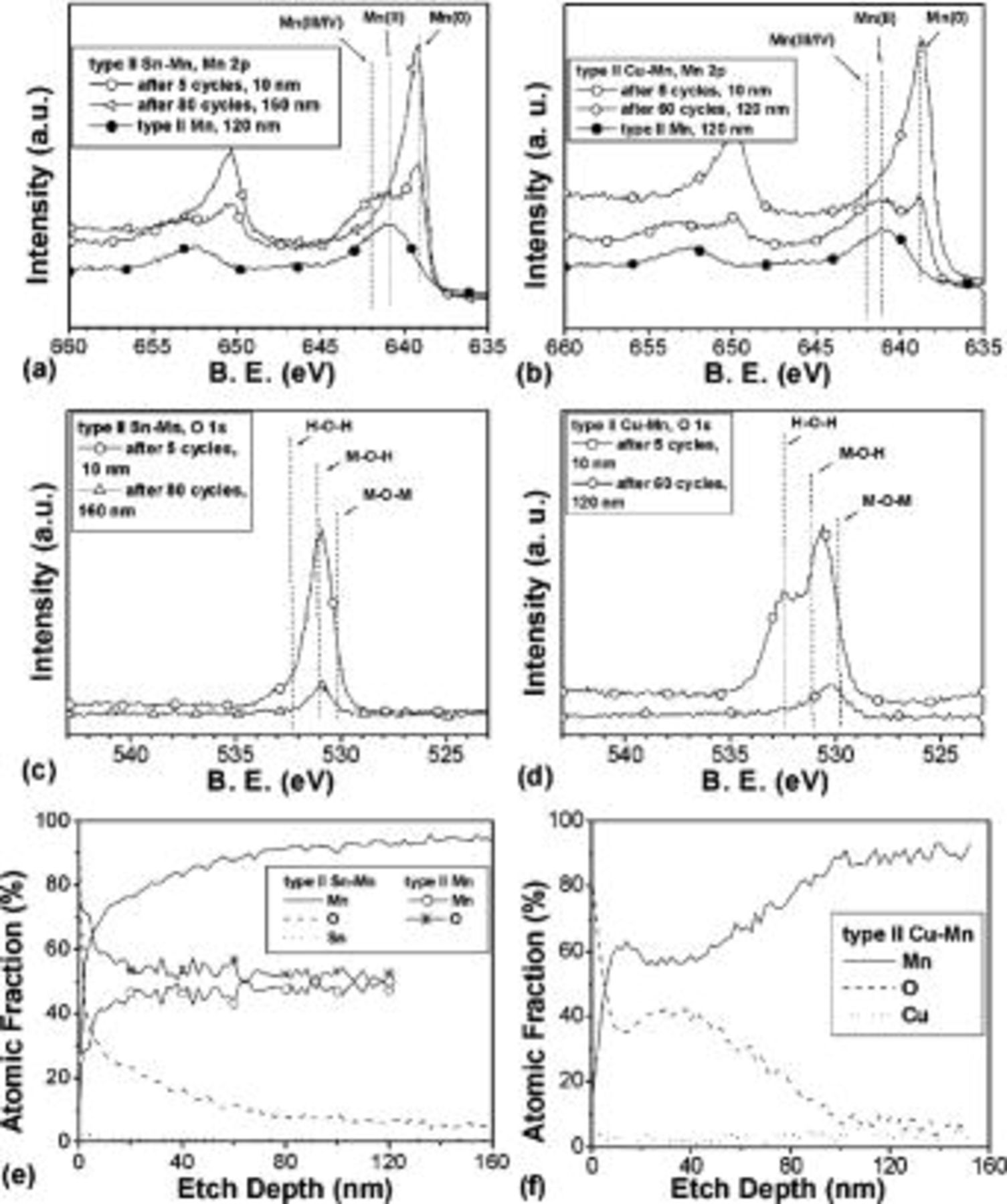
Figure 3a-d shows high resolution (HR) XPS scans of the Mn 2p and O 1s regions for type II Sn-Mn and Cu-Mn coatings at the surface and in the bulk, compared with type II Mn coatings. HR XPS scans of the Sn (Cu) regions were also collected but are not shown. The standard peak position of each chemical state is shown as dotted lines. In addition, the atomic percentage of Mn, Sn/Cu, and O at different depths in the coatings is shown in Fig. 3e,f.
Figure 3. High resolution XPS spectra of type II alloy coatings (see Fig. 4 in Ref. 11 for Mn): (a) Sn-Mn, Mn 2p, (b) Cu-Mn, Mn 2p, (c) Sn-Mn, O 1s; (d) Cu-Mn, O 1s, (e) Mn-O-Sn compositional depth profiles, and (f) Mn-O-Cu compositional depth profiles. (a), (b) and (f) also include for comparison the XPS spectrum and the compositional depth profile of type II Mn (from Ref. 11). Sn-Mn coatings are obtained from an electrolyte containing  (0.01 M),
(0.01 M),  (0.59 M), and
(0.59 M), and  (1 M) at 600 mA/cm2. Cu-Mn coatings are obtained from an electrolyte containing
(1 M) at 600 mA/cm2. Cu-Mn coatings are obtained from an electrolyte containing  (0.005 M),
(0.005 M),  (0.59 M), and
(0.59 M), and  (1 M) at 600 mA/cm2. pH is 2.6-2.8.
(1 M) at 600 mA/cm2. pH is 2.6-2.8.
In amorphous (type II) Mn coatings, the Mn:O atomic ratio reduced from 26:74 at the surface to 39:61 at 6 nm under the surface. Below 6 nm, the composition and chemical states did not depend much on depth (Fig. 3e), Mn is predominantly in the +2 and +3 valence states (Fig. 3a), and Mn(0) is only 6-8 atom % in the bulk. The oxygen content in type II Sn-Mn coatings was greatly reduced, and the metal content increased to a relevant extent as a consequence of the addition of a small amount of  in the solution (Fig. 3e). More precisely, the Mn:Sn:O atomic ratio changed from 55:3:42 at the surface (about 2 nm deep) to 93:1:6 after 80 etching cycles (about 160 nm). The compositional gradient observed in type II Sn-Mn coatings is different from that of pure Mn coatings. O content reduced sharply in the first several nanometers of naturally oxidized layer, and then decreased gradually. By deconvoluting the O 1s peak using three components (corresponding to H-O-H, Mn-O-H, and Mn-O-Mn),25 most of the oxygen was found to exist in the form of hydroxide (66 atom %), though its overall content was very low in the bulk. By deconvoluting the Mn 2p peak using three components (corresponding to Mn(0), Mn(II), and Mn(III/IV)),25 near the surface (in the naturally oxidized layer) the coating was rich in Mn(II-IV), while the bulk was rich in Mn(0) (>60 atom %) (Fig. 3a). Sn was in an oxidized form at the surface12 and "quasi-metallic" form28
29 in the bulk (>20 nm deep). The relative concentration of Sn was kept under 1 atom % below 10 nm depth and throughout the coating.
in the solution (Fig. 3e). More precisely, the Mn:Sn:O atomic ratio changed from 55:3:42 at the surface (about 2 nm deep) to 93:1:6 after 80 etching cycles (about 160 nm). The compositional gradient observed in type II Sn-Mn coatings is different from that of pure Mn coatings. O content reduced sharply in the first several nanometers of naturally oxidized layer, and then decreased gradually. By deconvoluting the O 1s peak using three components (corresponding to H-O-H, Mn-O-H, and Mn-O-Mn),25 most of the oxygen was found to exist in the form of hydroxide (66 atom %), though its overall content was very low in the bulk. By deconvoluting the Mn 2p peak using three components (corresponding to Mn(0), Mn(II), and Mn(III/IV)),25 near the surface (in the naturally oxidized layer) the coating was rich in Mn(II-IV), while the bulk was rich in Mn(0) (>60 atom %) (Fig. 3a). Sn was in an oxidized form at the surface12 and "quasi-metallic" form28
29 in the bulk (>20 nm deep). The relative concentration of Sn was kept under 1 atom % below 10 nm depth and throughout the coating.
The addition of  had a similar effect on the coating chemical composition. For type II Cu-Mn films, the Mn:Cu:O atomic ratio changed from 61:3:36 at 10 nm depth to 90:3:7 at 120 nm depth (Fig. 3f). However, the compositional gradient for the Cu-Mn coating was different from the others; a relatively thick oxide layer was observed, with a nonmonotonous variation of oxygen content with thickness. As in Sn-Mn, the bulk content of O in amorphous Cu-Mn coatings was much lower than in type II Mn coatings, and even lower than that in metallic, crystalline type I Cu-Mn coatings. Detailed investigation of O 1s spectra indicated that the small amount of oxygen inclusions was in the form of
had a similar effect on the coating chemical composition. For type II Cu-Mn films, the Mn:Cu:O atomic ratio changed from 61:3:36 at 10 nm depth to 90:3:7 at 120 nm depth (Fig. 3f). However, the compositional gradient for the Cu-Mn coating was different from the others; a relatively thick oxide layer was observed, with a nonmonotonous variation of oxygen content with thickness. As in Sn-Mn, the bulk content of O in amorphous Cu-Mn coatings was much lower than in type II Mn coatings, and even lower than that in metallic, crystalline type I Cu-Mn coatings. Detailed investigation of O 1s spectra indicated that the small amount of oxygen inclusions was in the form of  where x increases and n decreases with depth. In addition, an obvious Mn(0) peak (Fig. 3b) showed that Mn(0) is the main form of Mn (over 60% of total Mn). Cu was in the metallic form throughout and was distributed uniformly.
where x increases and n decreases with depth. In addition, an obvious Mn(0) peak (Fig. 3b) showed that Mn(0) is the main form of Mn (over 60% of total Mn). Cu was in the metallic form throughout and was distributed uniformly.
Summarizing, at high current density electrodeposited manganese coatings grew predominantly as Mn oxyhydroxides, probably due to a local pH increase at the interface caused by strong hydrogen evolution, which led to metal hydrolysis (Eq. 1) and then to the further oxidation of metal hydroxides (Eq. 2)
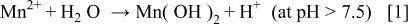
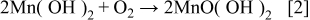
These reactions and consequently the inclusion of oxygen in amorphous Mn coatings seem to be inhibited by the addition of  or
or  ions, perhaps due to the presence of different species that adsorb on the electrode preferentially to
ions, perhaps due to the presence of different species that adsorb on the electrode preferentially to  avoiding Mn-oxyhydroxide incorporation, and/or to the possibility of different chemical equilibria in the electrolyte bulk. These phenomena result in a dramatic change in the chemical states of amorphous Mn coatings, and hence in the production of essentially metallic amorphous Mn (O content below 7 atom %, other metals below 1 (Sn) and 3 (Cu) atom %). The crystalline to amorphous transition in Mn and Mn alloys is due to the extremely high nucleation rate induced by the high applied overvoltage and by the strong hydrogen evolution and probable incorporation, that cannot be detected by XPS. Although the mechanism for the observed change in chemical states is not clear at this time, we believe that this effect may be important for other codeposition systems, as well as for the electrodeposition of other active metals.
avoiding Mn-oxyhydroxide incorporation, and/or to the possibility of different chemical equilibria in the electrolyte bulk. These phenomena result in a dramatic change in the chemical states of amorphous Mn coatings, and hence in the production of essentially metallic amorphous Mn (O content below 7 atom %, other metals below 1 (Sn) and 3 (Cu) atom %). The crystalline to amorphous transition in Mn and Mn alloys is due to the extremely high nucleation rate induced by the high applied overvoltage and by the strong hydrogen evolution and probable incorporation, that cannot be detected by XPS. Although the mechanism for the observed change in chemical states is not clear at this time, we believe that this effect may be important for other codeposition systems, as well as for the electrodeposition of other active metals.
The increase in the metallic character of amorphous Mn electrodeposits induces also relevant changes in their properties, as discussed in the following.
Tribological and mechanical properties.—
The friction coefficients extracted from nanoscratch experiments, as well as nanohardness and reduced modulus as deduced from Hysitron experiments for type II Mn, Sn-Mn, and Cu-Mn coatings are shown in Table I, where they are compared with reference electrodeposited Cd and crystalline (type I) Mn coatings. All coatings exhibit a friction coefficient lower than Cd, which is desirable in practical uses. Type II Sn-Mn coatings exhibit higher nanohardness than both Cd and Mn, and in addition possess high modulus. Incorporation of Cu into the Mn lattice on the contrary decreases both hardness and modulus. The resistance to plastic penetration,  30 is also shown in Table I, showing that Cu-Mn alloys in particular present outstanding mechanical properties for use as protective coatings of steel.
30 is also shown in Table I, showing that Cu-Mn alloys in particular present outstanding mechanical properties for use as protective coatings of steel.
Table I.
| Mechanical and tribological properties of Mn, Sn-Mn, and Cu-Mn coatings. | ||||||
|---|---|---|---|---|---|---|
| ECDCd | Type IMn | Type IIMn | Type IISn-Mn | Type ICu-Mn | Type IICu-Mn | |
| FrictionCoefficient | 0.8 | — | 0.5 | 0.35 | 0.7 | 0.5 |
| Nanohardness H (GPa) | 0.3 | 0.4 | 4.0 | 7.3 | 0.1 | 0.5 |
Reduced modulus 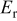 (GPa) (GPa) | 151.9 | 31.1 | 95.3 | 162 | 10.5 | 32.9 |
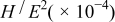 | 0.13 | 4.1 | 4.4 | 2.8 | 9.1 | 4.6 |
Corrosion resistance.—
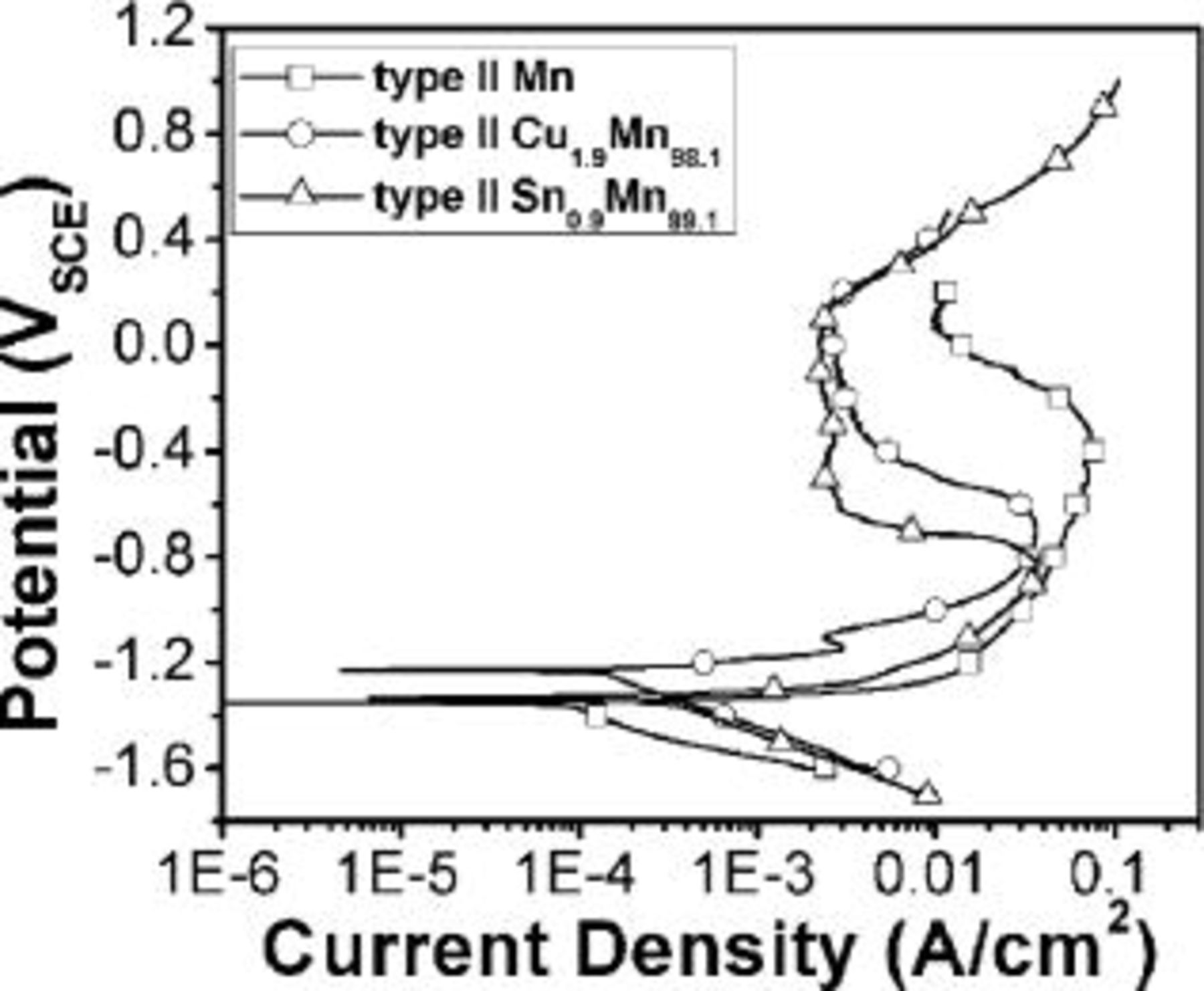
Anodic potentiodynamic corrosion experiments were performed for type II Mn, Sn-Mn, and Cu-Mn coatings, as shown in Fig. 4. In contrast with Mn, coatings containing small amounts of Sn or Cu show a pseudopassive region at anodic potentials in 3% NaCl solution at pH 3.0. In addition, the open-circuit potential (OCP) of Cu-Mn is slightly higher than Sn-Mn, probably as a consequence of the higher redox potential of Cu and the higher Cu content with respect to Sn. The OCP of these coatings (around −1.25  is more active than that of low carbon steels. It is thus anticipated that these coatings may provide sacrificial protection while presenting low chemical reactivity and thus slow dissolution in corrosive media.
is more active than that of low carbon steels. It is thus anticipated that these coatings may provide sacrificial protection while presenting low chemical reactivity and thus slow dissolution in corrosive media.
Figure 4. Typical anodic potentiodynamic behavior of type II Mn, Sn-Mn, and Cu-Mn coatings in 3% NaCl solution (pH 3.0). Scan rate is 2 mV/s.
Conclusions
The addition of a small amount of  or
or  to an electrolyte for Mn electrodeposition induced profound changes in the appearance, composition, and properties of amorphous coatings obtained at high current density. In particular, essentially metallic Mn films containing small amounts of the metal additives were obtained in amorphous form. The coating mechanical and tribological properties, as well as the corrosion resistance, may be improved by addition of Sn or Cu in the electrolyte. The possibility of influencing the chemical state of electrodeposited reactive metals by use of small amounts of metal ions may in principle be observed in other electrodeposition systems and possibly have important applications.
to an electrolyte for Mn electrodeposition induced profound changes in the appearance, composition, and properties of amorphous coatings obtained at high current density. In particular, essentially metallic Mn films containing small amounts of the metal additives were obtained in amorphous form. The coating mechanical and tribological properties, as well as the corrosion resistance, may be improved by addition of Sn or Cu in the electrolyte. The possibility of influencing the chemical state of electrodeposited reactive metals by use of small amounts of metal ions may in principle be observed in other electrodeposition systems and possibly have important applications.
Acknowledgments
This work was supported by DOD-SERDP under contract DACA72-99-P-0201, and by grant no. NSF-DMR-0093154. The use of MRSEC shared facilities was supported by NSF-DMR 9809423.