Article Text
Abstract
Peripheral neuropathy is a common finding in patients with complex inherited neurological diseases and may be subclinical or a major component of the phenotype. This review aims to provide a clinical approach to the diagnosis of this complex group of patients by addressing key questions including the predominant neurological syndrome associated with the neuropathy, for example, spasticity, the type of neuropathy and the other neurological and non-neurological features of the syndrome. Priority is given to the diagnosis of treatable conditions. Using this approach, we associated neuropathy with one of three major syndromic categories: (1) ataxia, (2) spasticity and (3) global neurodevelopmental impairment. Syndromes that do not fall easily into one of these three categories can be grouped according to the predominant system involved in addition to the neuropathy, for example, cardiomyopathy and neuropathy. We also include a separate category of complex inherited relapsing neuropathy syndromes, some of which may mimic Guillain-Barré syndrome, as many will have a metabolic aetiology and be potentially treatable.
- neuropathy
- neurogenetics
Statistics from Altmetric.com
Introduction
Inherited peripheral neuropathies can occur as a ‘pure’ neuropathy or as part of a more complex neurological or multisystem disorder. Charcot-Marie-Tooth disease (CMT) and the related neuropathies, distal hereditary motor neuropathy (HMN) and hereditary sensory neuropathy (HSN) are the classical ‘pure’ neuropathies. They commonly present with a characteristic phenotype of a length-dependent, isolated neuropathy progressing over decades.1
In the second group of disorders, where neuropathy is part of a more complex disease, the diagnosis is more challenging. In addition to well-recognised causes of these complex neuropathies (eg, Friedreich’s ataxia), next-generation sequencing (NGS) has identified an ever expanding number of causative genes. These include genes that were originally thought to cause other neurological syndromes (eg, Atlastin 1 was originally identified as causing hereditary spastic paraparesis but also causes HSN2 3) and complex inherited diseases (such as Krabbe’s disease) which can present with a CMT-like neuropathy, and in which the neuropathy may remain as the only manifestation of the disease.4
This review aims to provide a comprehensive list of the complex inherited neuropathy syndromes and an approach to diagnosis that is based on the major clinical features, for example, ataxia plus neuropathy or spasticity plus neuropathy as a pragmatic framework for clinical practice. While aimed at adult neurologists, this review includes some childhood diseases, including forme frustes that have adult presentations, such as recessive mutations in IGHMBP2, which cause spinal muscular atrophy with respiratory distress (usually a fatal childhood disease), but can cause adult-onset-recessive CMT2.5
Comprehensive disease list generation
To identify complex inherited diseases associated with a peripheral neuropathy, we compiled the authors’ lists and performed a PubMed search (in September 2016) for all publications describing a syndromic inherited neuropathy. The following search syntax was used: (‘peripheral neuropathy’) AND (‘inherit*’ OR ‘genetic’) NOT (‘Charcot Marie Tooth’). All papers which described an inherited neuropathy in conjunction with other clinical features were included. For each condition identified in the search, the presence of a neuropathy was confirmed by reviewing the original clinical description and neurophysiology. Multiple reviews exist, including our recent review, for the ‘pure’ neuropathies,1 that is, CMT and related disorders so these will not be covered except for selected cases that we feel are more appropriately classified as a complex inherited neuropathy syndrome.
A clinically based approach to the complex inherited neuropathy syndromes
Even knowing where to start in the diagnostic evaluation of a patient with a complex inherited neuropathy syndrome can feel daunting. This is in part due to the large number of potentially causative genes but also to the rarity of these diseases, some of which are so rare (as small as single families) that even an experienced clinician is unlikely to have encountered them. The situation is further complicated by the fact that the neuropathy can be a major feature of the syndrome or largely masked by other clinical features. In this review, we included all inherited syndromes in which neuropathy has been described even if it is only a minor feature and present in a minority of patients (eg, SPG3A due to dominant mutations in Atlastin 1 2). This is because the most prominent phenotype of a syndrome may vary for a particular genetic condition. For example, in a patient with Friedreich’s ataxia, a sensory neuropathy may be the main presenting feature whereas in others it may be a cerebellar ataxia. Having identified 157 complex inherited diseases with a neuropathy, we addressed the following four questions.
What is the predominant neurological phenotype?
Is the neuropathy predominantly motor or sensory and is the neuropathy clearly axonal or are the conduction velocities slow?
What are the other neurological and non-neurological features of the disease?
Is the disease treatable?
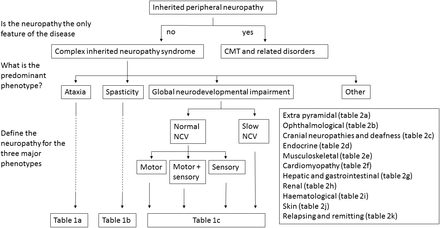
This strategy allowed us to develop a diagnostic approach based on the identification of the predominant phenotype (see figure 1). For the majority of conditions, this can be divided into the following three major neuropathy associated categories: (1) ataxia, (2) spasticity and (3) global neurodevelopmental impairment. For the complex neuropathy syndromes that do not fall easily into one of these three categories, we used 11 other phenotypic categories: (1) extrapyramidal features, (2) ophthalmic disease, (3) cranial neuropathies and deafness, (4) endocrinopathy, (5) musculoskeletal disease/myopathy, (6) cardiomyopathy, (7) hepatic and gastrointestinal (GI) disease, (8) renal failure, (9) haematological and immunological diseases and (10) skin and connective tissue features. We also include a separate category of complex inherited relapsing neuropathy syndromes, some of which may mimic Guillain-Barré syndrome. This is an important group of diseases as many have a metabolic aetiology and are often treatable if identified early in the disease course.
A diagnostic approach for patients with complex inherited neuropathy syndromes. The first step is to decide if the neuropathy is the sole feature of the disease, that is, Charcot-Marie-Tooth disease or the related disorders, hereditary motor neuropathy and hereditary sensory neuropathy or if it is part of a more complex syndrome. In patients in whom there are additional features, the majority will fall into one of the three major categories, ataxia, spasticity or global neurodevelopmental delay. Further classification is based on the features of the neuropathy and the reader is directed to the appropriate table for the list of possible disease genes. A proportion of patients will not fall into one of these three categories (‘other’) and in this scenario, further classification is based on additional clinical features, for example, extrapyramidal disease, and the reader is directed to the appropriate table for a list of disease genes. NCV, nerve conduction velocity.
The introduction of NGS (either whole-genome, whole-exome or gene-specific panels) into clinical practice offers great promise for diagnosing complex inherited neuropathy syndromes.6 The ability to sequence all known disease genes (>150 genes can cause a neuropathy as part of a complex inherited syndrome and almost 100 additional genes cause a form of CMT), however, is not a panacea for diagnosing this group of patients. The challenge, therefore, is the interpretation of the large number of novel variants identified in known disease genes for each individual. Knowing the phenotypes of the inherited complex neuropathy syndromes is one key to interpreting these variants. Because the prognosis of rare, treatable complex inherited neuropathy syndromes depends on early diagnosis, we have priorised these diseases.
The cost of NGS in the form of disease-specific panels is often cheaper than targeted Sanger sequencing of individual genes. We therefore recommend proceeding to disease-specific panels (eg, ataxia, spasticity, developmental delay) in the first instance. The only exception is for the ataxia and neuropathy syndromes where we recommend targeted testing for repeat expansion diseases first in cases with an appropriate phenotype (eg, Friedreich’s ataxia, FXTAS, SCA1, 2, 3, 10, 12). With advances in NGS, it is likely that disease- specific panels will eventually be able to reliably detect repeat expansions.
Major complex inherited neuropathy categories
Ataxia and neuropathy syndromes (table 1a)
In the diagnostic evaluation of patients with neuropathy and cerebellar ataxia, we propose an initial screen for treatable causes followed by categorisation into whether the neuropathy is sensory and motor, predominantly motor or predominantly sensory as well as those with slow nerve conduction velocities (SNCV) (less than acceptable for axonal loss). Patients without an obvious initial diagnosis and a neuropathy with either normal or slow conduction velocities should have blood levels of phytanic and pristanic acid (Refsum’s disease may be treated with dietary modification and plasma exchange7) as well as very long chain fatty acids and lysosomal enzymes measured (allogenic bone marrow transplantation may be effective in some peroxisomal and lysosomal storage diseases (eg, adrenoleukodystrophy)8 9). Vitamins E and B12, including methylmalonic acid and homocysteine (to screen for functional vitamin B12 deficiency), should be measured as deficiencies may cause an ataxia and neuropathy phenotype and may be treatable.10 Finally, although rare, plasma cholestanol for cerebrotendinous xanthomatosis is an important disease to identify early in the disease course as it is preventable with dietary modification and treatment with chenodeoxycholic acid. Clues to this diagnosis include the combination of diarrhoea, cataracts or infantile jaundice.11
A summary of the complex inherited neuropathy syndromes with one of the three major core clinical phenotypes of ataxia, spasticity or global neurodevelopmental impairment
Most patients with ataxia and a neuropathy will have an axonal neuropathy with normal nerve conduction velocities and reduced distal amplitudes. A motor-predominant axonal neuropathy or neuronopathy is rare in the ataxia neuropathy syndromes but is seen in SCA2 and SCA36.12 SCA2 is a trinucleotide repeat disease and therefore may not be identified on NGS. Interestingly, an expansion size of between 30 and 35 repeats is associated with amyotrophic lateral sclerosis,13 whereas larger expansion sizes will cause a combined neuropathy, ataxia syndrome often with slow saccadic eye movements, tremor and occasionally an extrapyramidal disorder that may mimic multiple system atrophy.14 SCA36 presents as a late-adult-onset ataxic syndrome with a distal motor neuropathy and bulbar involvement.12 It is caused by a hexanucleotide expansion.
Ataxia and a sensory axonal neuropathy is the most common combination caused by recessive mutations in a variety of genes, usually with disease onset in the first decade. The sensory neuropathy may contribute to the manifestations of the ataxia. Friedreich’s ataxia, due to a trinucleotide repeat expansion in the FDRA gene, is the most common form.15 Ataxia telangiectasia, early-onset ataxia with oculomotor apraxia and hypoalbuminemia (EAOH/aprataxin) and spinocerebellar ataxia, autosomal-recessive 1 (SCAR1/senataxin) may also cause a sensory ataxic neuropathy syndrome similar to Friedreich’s ataxia often in association with a raised serum α-fetoprotein level. Distinguishing clinical features include the presence of cardiomyopathy in Friedreich’s ataxia, ‘oculomotor apraxia’ in EAOH and SCAR1 and chorea, conjunctival telangiectasia and the susceptibility to infections and malignancies in ataxia telangiectasia.15
The autosomal-dominant SCAs 1, 3, 7, 10 and 12 may all cause a sensory and motor axonal neuropathy. They are all due to repeat expansions and may therefore require targeted genetic testing. Phenotypic clues to the individual SCAs include extrapyramidal signs and ophthalmoplegia in SCA3,16 pigmentary macular degeneration in SCA7 and prominent tremor in SCA12.17 18
A neuropathy with SNCV is rare in patients with an ataxia neuropathy syndrome. The most common by far is autosomal-recessive spastic ataxia of Charlevoix-Saguenay (ARSACS) due to recessive mutations in the SACS gene, which encodes the molecular chaperone protein, DNAJC29.19 Neuropathy may be the presenting issue and the most prominent clinical finding (see online supplementary file 1 for an example of the neurophysiology).19 In addition to ataxia and a neuropathy, patients may develop a myelopathy and in rare cases seizures. Ataxia, combined peripheral and cerebellar, with hearing loss and diabetes mellitus (APCHD), due to recessive mutations in another heat shock protein DNAJC3, may also cause an SNCV neuropathy.20 Finally, polyneuropathy, hearing loss, ataxia, retinitis pigmentosa and cataracts (PHARC) syndrome is also a rare cause.21
Supplementary file 1
Further diagnostic clues can be obtained by MRI of the brain. This may identify white matter changes that are highly suggestive of specific diagnoses for some ataxia neuropathy syndromes—high signal of the deep white matter tracts of the brain and dorsal columns and lateral corticospinal tracts due to recessive DARS2 mutations,22 T2 high signal of the middle cerebellar peduncles in Fragile X tremor ataxia syndrome23 and white matter signal abnormalities and bilateral dentate nuclei lesions of the cerebellum in cerebrotendinous xanthomatosis24 (see figure 2).
Examples of central nervous system MRI that may assist in the diagnostic evaluation of patients with peripheral neuropathy as part of a complex inherited disease. (Ai) shows coronal Fluid-attenuated Inversion Recovery (FLAIR) and (Aii) sagittal T1w images from a patient with SPG11 demonstrating a thin corpus callosum and cerebellar hypoplasia. (B) shows an axial T2w image of a patient with metachromatic leukodystrophy in which there is bilateral confluent white matter signal abnormality with cerebral volume loss. (C) is an axial T2w image from a patient with fragile X tremor ataxia syndrome and demonstrates bilateral signal change in the cerebellar peduncle (arrows) and pontine, peduncular and cerebellar volume loss. (D) shows coronal FLAIR (Di) and axial T2w (Dii) images from a patient with adult-onset polyglycosan body disease demonstrating multifocal cerebral white matter lesions. (E) shows axial T2w (Ei) and coronal T1w (Eii) images from a patient with cerebrotendinous xanthomatosis demonstrating symmetrical signal change involving the peridentate white matter of both cerebellar hemispheres. (F) shows a selection of images from a patient with leukoencephalopathy with brainstem and spinal cord involvement and raised lactate. (Fi) and (Fii) are sagittal and axial images of the cervical cord demonstrating longitudinally extensive T2 hyperintense signal change involving the dorsal and lateral columns. The characteristic brainstem signal change (red arrows) in an axial T2w image is shown in (Fiii). (Fiv) shows the small lactate peak (red arrow) detected using localised 1 hour magnetic resonance spectroscopy. (G) shows axial T2w (Gi and Gii) and coronal FLAIR (Giii) images from a patient with Krabbe disease. The images show signal change involving the long tracts and deep grey matter. (H) shows axial T2w (Hi) and susceptibility weighted (SWI) (Hii and Hiii) images from a patient with neurodegeneration and brain iron accumulation. Although the T2w image looks normal, the SWI images show increased susceptibility from abnormal mineralisation in the superficial and deep cortical grey matter.
Spasticity and neuropathy syndromes (table 1b)
The initial diagnostic evaluation of patients with spastic neuropathy syndromes without an obvious cause should include measurement of vitamin B12, methylmalonic acid and folate, phytanic and pristanic acid (alpha-methylacyl-CoA racemase deficiency (AMACR)), very long chain fatty acids and lysosomal enzymes. In addition, one should have a low threshold for performing MRI of the spinal cord to find a structural cause of myelopathy.
After screening for treatable spastic neuropathy syndromes, we suggest that the next step is to define the underlying neuropathy. Unlike the neuropathy ataxia syndromes, a motor axonal neuropathy is a feature of some hereditary spastic paraplegias (HSP) which may present as either an HSP or distal HMN. In SPG20 (Troyer syndrome), a distal motor neuropathy is seen in combination with spasticity, short stature and learning difficulties.25 SPG9A and SPG9B are also characterised by spastic paraplegia, learning difficulties and a distal motor neuropathy in addition to cataracts and skeletal abnormalities.26 SPG17 (Silver syndrome) is an autosomal-dominant disorder due to BSCL2 mutations and is a relatively common cause of HSP and distal motor neuropathy predominantly affecting the upper limbs.27 Distal spinal muscular atrophy type 2 (DSMA2), due to recessive mutations in SIGMAR1, causes a similar phenotype but preferentially affecting the extensor muscles of the forearm.28 The combination of motor neuropathy and spastic paraplegia has also been reported in SPG3929 and SPG12 (MMR and AMR personal observation in a recessive case).
SPG10 is a more common cause of spasticity and a mixed motor and sensory axonal neuropathy and may be complicated by cognitive decline and parkinsonism.30
A pure sensory axonal neuropathy and spasticity is less common but in combination with an ulceromutilating phenotype suggests mutations in CCT5 (HSN with spastic paraplegia),31 ARL6IP1 (SPG61) and rarely ATL1 (SPG3A).3 32
A neuropathy with SNCV in association with spasticity is also rare but reported in ARSACS and PHARC syndrome (see ataxia neuropathy section).21 33 In addition, SNCV have been described in the neuropathy associated with the peroxisomal disorder, AMACR.34
Bladder involvement, which is probably not uncommon in many kinds of HSPs, may be a prominent feature of adult polyglycosan body disease,35 adrenomyeloneuropathy and SPG5A.36 37
MRI of the brain can provide important diagnostic clues (see figure 2). Periventricular white matter lesions suggestive of multiple sclerosis may be seen in adult-onset polyglycosan body disease and SPG5A.35 36 Some cases of neurodegeneration with brain iron accumulation diseases may present as a spasticity neuropathy syndrome (eg, mutations in PLA2G6, C19orf12/SPG43); MRI shows iron deposition in the basal ganglia.38 39 Finally, a group of recessive spastic paraplegia genes associated with a thin corpus callosum on MRI have recently been identified as a cause of neuropathy spasticity syndromes. SPG11 is the most common of these syndromes and presents with spastic paraplegia, cognitive decline, sensory and motor axonal neuropathy and often weight gain40; patients with SPG15 have a similar phenotype but with pigmentary maculopathy41; SPG46 is a similar disease to SPG11 but with cataracts.42
Global neurodevelopmental impairment and neuropathy syndromes (table 1c)
Achieving a diagnosis is more difficult in this phenotypic category. Most are rare. Characterising the phenotype may be challenging as there is a broad range of phenotypes including spasticity, ataxia, cardiomyopathy, endocrine and GI dysfunction and dermatological manifestations, further complicated by developmental delay. Nevertheless, with the advent of NGS, it is likely that milder forms of these diseases will be described and an awareness of the key clinical features may assist in diagnosis. Few are currently treatable, but screening for metachromatic leukodystrophy and Krabbe disease is recommended as both may be treatable disorders. In addition, there are clinical trials for Aicardi-Goutieres syndrome and giant axonal neuropathy (ClinicalTrials.gov identifier NCT02362453 and NCT02362438).
As for the spastic and ataxic neuropathy syndromes, defining the type of neuropathy can be helpful in achieving a genetic diagnosis. A pure motor axonal neuropathy as part of a complex neurodevelopmental syndrome is seen with mutations in DYNC1H1 and BICD2.43 44 The two conditions are almost identical and can present as arthrogryposis predominantly affecting the lower limbs. Other causes of a motor neuropathy/neuronopathy in this group include pontocerebellar hypoplasia type 1 B and hexosaminidase A and B deficiency.45 46
Global developmental delay is a relatively common finding in several of the congenital insensitivity to pain syndromes (eg, recessive CTLCL1 mutations47), although in some cases the sensory nerve conduction studies may be normal despite significant ulceromutilation as is seen with recessive loss-of-function SCN9A mutations.48 Recessive mutations in TECPR2 are a rare cause of a sensory and autonomic neuropathy with global developmental delay, in which patients also experience chronic respiratory disease, apnoeas and seizures.49
A peripheral neuropathy with SNCV is a more common finding among this group of diseases and includes the lysosomal storage diseases metachromatic leukodystrophy and Krabbe disease. Other causes include HLD5 (leukodystrophy, hypomyelination and congenital cataracts),50 congenital disorders of glycosylation (recessive PMM2 mutations),51 Andermann’s syndrome (agenesis of the corpus callosum and peripheral neuropathy),52 Cockayne syndrome,53 Leigh’s syndrome due to SURF1 and MFF mutations,54 55 the complex infantile-onset IMNEPD (complex neurodegeneration in the context of hearing loss and pancreatic insufficiency)56 and Aicardi-Goutières syndrome which is an inflammatory disease that presents as an inflammatory syndrome and may respond partially to immunosuppression.57
MRI of the brain can be useful for directing genetic investigations in this group of patients (see figure 2). The detection of a leukodystrophy is seen in many of the lysosomal storage disorders and in metachromatic leukodystrophy, if characteristic, should prompt further investigations in the face of low normal aryl sulfatase activity to ensure that a sulfatide activator protein deficiency is not missed.58 Other diseases associated with white matter findings pointing to a possible leukodystrophy include Krabbe disease, congenital disorders of glycosylation, HLD5, giant axonal neuropathy and Aicardi-Goutières syndrome.50 51 59
Other MRI findings may also provide clues to the genetic aetiology—agenesis of the corpus callosum in Andermann’s syndrome,52 cerebral dysgenesis in the severe cerebral dysgenesis, neuropathy, ichthyosis and palmoplantar keratoderma (CEDNIK) syndrome,60 pontocerebellar hypoplasia in PCH 1B and 9 (where it is also associated with agenesis of the corpus callosum)w61 w62 and iron deposition in NB12A due to PLA2G6 38 mutations, and striatal necrosis in Aicardi-Goutières syndrome.57
Other complex inherited neuropathy categories
Extrapyramidal disease and neuropathy syndromes (table 2a)
Peripheral neuropathy is a rare association with extrapyramidal disease and is most commonly seen in the context of mitochondrial disease due to either nuclear or mitochondrial DNA mutations. The classical SANDO syndrome of sensory axonal neuropathy, dysarthria and ophthalmoplegia can be associated with parkinsonism.w63 Chorea and dystonia in the context of a motor predominant neuropathy is seen with both Chorea acanthocytosis and McLeod’s syndrome.w64 Finally, recessive mutations in HSJ1 and both dominant and recessive mutations in LRSAM1 (proteins involved in the ubiquitin proteosome system) present with late-onset CMT2 but may develop Parkinson’s disease later in life.w65 w66
A summary of the complex inherited neuropathy syndromes with one of the minor 10 clinical phenotypes associated with neuropathy
Ophthalmological and neuropathy syndromes (table 2b)
Performing a thorough ophthalmological examination to detect external ophthalmoparesis, optic atrophy, retinitis pigmentosa and cataracts can be useful in refining the potential genetic diagnosis of a complex inherited neuropathy syndrome.
The combination of severe optic atrophy and a mild and predominantly sensory axonal neuropathy is suggestive of mutations in either OPA1 or OPA3.w67 w68 These patients often have other clinical features including pseudo-obstruction, deafness, extrapyramidal signs and progressive external ophthalmoplegia. Mutations in MFN2, the cause of CMT2A, may also cause optic atrophy and an axonal neuropathy but in almost all cases the neuropathy predominates.w69
Retinitis pigmentosa is a relatively common feature among the complex neuropathy syndromes, particularly disorders of mitochondria (eg, Kearns-Sayre and NARP syndromes) and the peroxisome (Refsum’s and related diseases including AMACRD).7 34 w70 In addition, retinitis pigmentosa is also a feature of several other rare conditions including PHARC syndrome and the congenital disorders of glycosylation.21 51 Most importantly, it is a feature of a treatable (high doses of B12) genetic B12 deficiency syndrome (MMACHC), in which vitamin B12 plasma levels are normal but the downstream metabolites methylmalonic acid and homocysteine are elevated.w71
Cataracts are common in the general population but are helpful diagnostically if present in young patients. Although present in several conditions, for example, PHARC, congenital cataracts facial dysmorphism and neuropathy (CCFDN) and HLD5,21 50 w72 most importantly they are a feature of the treatable disease cerebrotendinous xanthomatosis and their presence should prompt testing of plasma cholastenol levels.11
Cranial neuropathies and deafness (table 2c)
Bilateral facial weakness and bulbar palsy is an uncommon phenotype in the complex neuropathy syndromes and strongly suggestive of spinal bulbar muscular atrophy (Kennedy’s disease) or Brown-Vialetto-Van Laere (BVVL) syndromes.w73 w74 Of these, BVVL is an important diagnosis not to miss. It is due to recessive mutations in one of two riboflavin transporters and both forms appear to respond to riboflavin supplementation.w74 w75 BVVL is almost always associated with deafness. In BVVLS2, patients often present with a sensory ataxic neuropathy whereas in BVVLS1 it is predominantly a motor neuronopathy. Some but not all patients with BVVLS2 may have an abnormal plasma acyl carnitine profile.w74
Progressive external ophthalmoplegia is the the most common disorder of cranial musculature and is seen with both nuclear and mitochondrial DNA mutations.w76 The presence of Duane syndrome, a congenital and non-progressive strabismus with a mild sensory and motor axonal neuropathy is seen with dominant mutations in TUBB3.w77
Sensory neuronal deafness as part of a complex neuropathy syndrome is commonly, but not exclusively, seen with mitochondrial disorders. The presence of sensory neuronal hearing loss in combination with a myopathy, although most commonly seen with mitochondrial disease, is also a feature of a distal myopathy and neuropathy overlap syndrome caused by mutations in MYH14.w78 The combination of ataxia, demyelinating neuropathy and sensory neuronal hearing loss is common to both PHARC syndrome and ACPHD.20 21 Finally, although classified as an HSN, HSN1E is defined by the presence of sensory neuronal hearing loss in combination with dementia and, in some cases, narcolepsy.w79
Endocrinopathy and neuropathy syndromes (table 2d)
Although diabetes mellitus is a feature of mitochondrial disease and a number of other complex syndromes including APCHD and Kennedy’s disease, its high prevalence in the general population reduces its discriminatory value.20 w73 Adrenal insufficiency, however, is a useful diagnostic clue for adrenomyeloneuropathy but also the achalasia, hypo adrenalism, alacrima syndrome (AAAS).w80 Ambiguous genitalia in combination with global neurodevelopmental impairment and a mixed sensory and motor axonal neuropathy is seen in the gonadal dysgenesis with minifascicular neuropathy syndrome.w81
Musculoskeletal/myopathy and neuropathy syndromes (table 2e)
The presence of a myopathy in combination with a leukodystrophy, ataxia, global developmental delay and a sensory and motor peripheral neuropathy with SNCV is almost diagnostic of congenital disorder of glycosylation type 1A.51 A mild neuropathy with slow conduction velocities is also seen in merosin-deficient congenital muscular dystrophy but is not a dominant feature.w82 To date, a sensory and motor axonal peripheral neuropathy with giant axons has been described in myofibrillar myopathy due to recessive mutations in BAG3, but the clinical phenotype is dominated by the myopathy and cardiomyopathy.w83 Recessive mutations in lamin A/C are a cause of CMT2 in North Africa; however, dominant mutations in the same gene causing a limb girdle muscular dystrophy may rarely be associated with a sensory and motor axonal neuropathy.w84
To date, only a subclinical sensory axonal neuropathy has been described in patients with multiple acyl CoA dehydrogenase deficiency. It is possible that forms of the disease exist in which neuropathy is a more prominent feature. The disease is characterised by episodes of hypoglycaemia, acidosis and a lipid storage myopathy. Most importantly, it is responsive to riboflavin supplementation.w85
Finally, genes that were originally reported to cause distal HMN are now recognised to cause both a myopathy and motor neuropathy.w86 w87 This is most pronounced for patients with mutations in HSPB8 in whom the myofibrillar myopathy dominates the clinical picture.87
Cardiomyopathy and neuropathy syndromes (table 2f)
Cardiomyopathy is seen in a number of complex inherited neuropathy syndromes including myofibrillar myopathy due to BAG3 mutations, mitochondrial disease, Fabry disease, Friedreich’s ataxia and McLeod’s syndrome.
The presence of an acquired cardiomyopathy in adulthood in combination with a painful sensory and motor axonal neuropathy is highly suggestive of familial amyloid polyneuropathy. Although tissue confirmation of amyloid is important, in the correct clinical context, sequencing of the TTR gene is warranted as a number of old (liver transplantation) and new (tafamidis and diflunisal) treatments are available.w88 A significant minority of patients with TTR amyloidosis have been reported with an SNCV neuropathy mimicking CIDP (eg, see supplementary file 1).
Hepatic, GI and neuropathy syndromes (table 2g)
Recurrent episodes of acute liver failure in combination with a neuropathy is suggestive of mitochondrial disease and has been described with mutations in the nuclear genes DGUOK and MPV17 and can occur in autosomal-recessive spinocerebellar ataxia 21.w89 w90
Hirschsprung disease is a developmental disorder of the mesenteric plexus and, in combination with global developmental delay and a neuropathy with SNCV, is seen with dominant mutations in SOX10.w91 The association of Hirschsprung disease, global neurodevelopmental impairment and an axonal sensory and motor neuropathy is seen in Goldberg-Shprintzen megacolon syndrome due to recessive mutations in KIAA1279.w92
Pseudo obstruction is an increasingly recognised complication of mitochondrial disease and can be caused by a number of gene mutations including POLG, RRM2B and TPP.w89 In its most severe form, mitochondrial neurogastrointestinal encephalopathy (MNGIE) patients may present with a neuropathy resembling CMT or chronic inflammatory demyelinating polyneuropathy associated with severe GI disturbance and weight loss.w93 It is most commonly due to recessive mutations in the nuclear gene thymidine phosphorylase and can be screened for by testing for elevated levels of thymidine and deoxyuridine in plasma. The disease arises from a deficiency of the enzyme thymidine phosphorylase, which is expressed in platelets. Allogenic bone marrow and liver transplantation have been successfully employed as treatments for this condition.w89 w94
The combination of adult-onset refractory diarrhoea, sensory axonal neuropathy and dysautonomia is suggestive of familial amyloid polyneuropathy but also rarely mutations in the prion protein gene, PRNP.w95 In the latter it is associated with dementia but this often occurs late in the disease.
Renal failure and neuropathy syndromes (table 2h)
Renal failure is rare in the complex inherited neuropathy syndromes. Nephropathy is a feature of familial amyloid polyneuropathy but it is rare for patients with mutations in TTR to develop frank renal failure. Renal failure is also seen in Fabry disease, an X-linked disorder associated with a painful sensory and small fibre neuropathy, angiokeratoma, strokes and a cardiomyopathy.
An intermediate form of CMT due to dominant mutations in INF2, a gene expressed in the glomerulus and peripheral nerve, is associated with a focal segmental glomerulosclerosis.w96 In almost all cases, the degree of renal failure eventually requires renal replacement therapy. The recently described action myoclonus-renal failure syndrome is characterised by a progressive myoclonic epilepsy and renal failure beginning in the second decade of life and associated with a sensory and motor neuropathy with slow conduction velocities.w97
Haematological and immunological neuropathy syndromes (table 2i)
The combination of haematological abnormalities and a peripheral neuropathy is unique to a small number of syndromes. The most important to recognise are the disorders of cobalamin (B12) metabolism that result in functional B12 deficiency. The most common of this group of diseases is methylmalonic aciduria and homocystinuria, cb1C (MMACHC), which can cause a syndrome similar to subacute combined degeneration of the cord but also other haematological abnormalities including a form of vitamin B12-responsive thrombotic thrombocytopenic purpura.w71
Autosomal-recessive mutations in CD59, a glycoprotein present on the cell surface that prevents formation of the complement-mediated membrane attack complex, results in a combination of haemolytic anaemia, strokes and a relapsing remitting demyelinating neuropathy. Eculizumab, an inhibitor of the complement membrane attack complex, has been used successfully in one patient.w98
Chediak-Higashi syndrome is an immunodeficiency syndrome characterised by neutropenia and an increased risk of lymphoma. It is associated with a sensory and motor axonal peripheral neuropathy and has been treated with allogenic bone marrow transplantation in selected cases.w99
Skin and connective tissue and neuropathy syndromes (table 2j)
Photosensitivity is a rare symptom but in combination with a peripheral sensory and motor axonal neuropathy is suggestive of xeroderma pigmentosa (XP), a disease which is associated with developmental delay and an increased risk of cutaneous malignancy.w100 Patients with Cockayne syndrome also experience skin photosensitivity, but unlike XP, the neuropathy has SNCV and there is no increased risk of malignancy.53
Skin laxity is an uncommon sign but is seen in combination with a SNCV neuropathy in dominant FBLN5 mutations, and in combination with a mixed sensory and motor axonal neuropathy with recessive mutations of PLOD1 and dominant mutations in EMILIN1.w101 w102 It is important to recognise these two diseases as patients have an increased risk of large vessel injury and aneurysms and may need to enter an aneurysm screening programme.
Relapsing complex inherited neuropathy syndromes (table 2k)
This group of diseases are important to recognise as they are more likely to have an underlying metabolic defect and are often treatable. The acute porphyrias, including acute intermittent porphyria, coproporphyria and variegate porphyria, can present as an acute neuropathy mimicking Guillain-Barré syndrome.w103 In AIP, relapses are associated with abdominal pain and seizures whereas in variegate and coproporphyria there is skin photosensitivity. These diseases can be screened for acutely by testing for porphobilinogen in a light-protected sample of urine. Identification of acute porphyria is important as early treatment with glucose and haematin in patients with an acute axonal neuropathy may improve the prognosis.
Tyrosinemia can present similarly to acute intermittent porphyria. It is diagnosed by the detection of raised levels of succinylacetone in blood and urine. In the acute setting, it is treated with plasma exchange. Nitisinone, which prevents the formation of the toxic products malcylacetoacetic acid and fumarylacetoacetic acid, offers a long-term treatment.w104
Maple syrup urine disease has been reported as a cause of an acute axonal neuropathy mimicking Guillain-Barré syndrome and is treated with dietary reduction of protein intake.w105 Thiamine metabolism dysfunction syndrome 4 is a condition characterised by a progressive chronic axonal neuropathy superimposed by episodes of acute encephalopathy and paralysis following a febrile illness. Thiamine is an unproven but recognised treatment.w106
Conclusion
Although the advent of NGS means that it is now feasible to sequence all known complex inherited neuropathy genes in a practical time frame, an overview of the phenotypes is still required to be able to help decide which novel variants are benign, which are pathogenic and which disease genes may not have been comprehensively screened using current NGS platforms. Obtaining an accurate genetic diagnosis in these conditions can be of great benefit to patients and their families especially for genetic counselling and to prevent unnecessary investigations. In this rapidly growing field, the identification of those diseases that may respond to treatment will always be the top priority, particularly as the number of treatable conditions increases.
Acknowledgments
MMR, SSS, MES and DP are grateful to the National Institutes of Neurological Diseases and Stroke and office of Rare Diseases (U54NS065712) for their support.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- w61.↵
- w62.↵
- w63.↵
- w64.↵
- w65.↵
- w66.↵
- w67.↵
- w68.↵
- w69.↵
- w70.↵
- w71.↵
- w72.↵
- w73.↵
- w74.↵
- w75.↵
- w76.↵
- w77.↵
- w78.↵
- w79.↵
- w80.↵
- w81.↵
- w82.↵
- w83.↵
- w84.↵
- w85.↵
- w86.↵
- w87.↵
- w88.↵
- w89.↵
- w90.↵
- w91.↵
- w92.↵
- w93.↵
- w94.↵
- w95.↵
- w96.↵
- w97.↵
- w98.↵
- w99.↵
- w100.↵
- w101.↵
- w102.↵
- w103.↵
- w104.↵
- w105.↵
- w106.↵
Footnotes
Contributors AMR performed a literature search, analysed the literature, wrote the first draft including tables and figures. ASC, ALP-N and HD performed additional literature searches and drafted manuscripts. HC collected MRI images and drafted the legend for figure 2. DP, SSS and MES revised the manuscript. SSS performed an additional literature search to identify additional diseases. MMR came up with the theme for the review and revised the manuscruipt.
Funding The INC (U54NS065712) is a part of the NCATS Rare Diseases Clinical Research Network (RDCRN). RDCRN is an initiative of the Office of Rare Diseases Research (ORDR), NCATS, funded through a collaboration between NCATS and the NINDS. This research was also supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre. AMR is funded by a Wellcome Trust Postdoctoral Fellowship for Clinicians (110043/Z/15/Z). MMR is grateful to the Medical Research Council (MRC), MRC Centre grant (G0601943).
Competing interests None declared.
Provenance and peer review Commissioned; externally peer reviewed.