Abstract
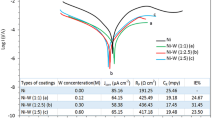
Nickel (Ni)–Tungsten (W) alloys were electrodeposited galvanostatically (at–10 mA cm–2) on copper substrate with 3 different W contents under the controlled hydrodynamic conditions and then the anodic dissolution behaviors of the alloys were observed by potentiodynamic polarization and electrochemical quartz crystal microbalance (EQCM) techniques. While the structure of the electrodeposited Ni–W alloy with low W content (15.90% W) was crystalline, that of the alloy with high W content (50.80% W) was nano-crystalline according to X-ray diffraction patterns. The increase in the W content of the electrodeposited Ni–W alloy resulted decrease at pH 3 and increase at pH 7 and 12.5 in the anodic currents of the alloy. The pH dependent dissolutions caused electrodeposited alloy surface to have W—enrichment at pH 3 and Ni—enrichment at pH 7 and 12.5. These observations indicated that the selective dissolution of Ni or W was the main mechanism in the anodic dissolution of the electrodeposited Ni–W alloys. The EQCM experiments conducted at pH 7 supported the presence of the selective dissolution mechanism that the anodic dissolution potential of W was 0.42 V lower than that of Ni in the electrodeposited Ni–W alloys.
Similar content being viewed by others

References
Tsyntsaru, N., Cesiulis, H., Donten, M., et al., Surf. Eng. 2012, vol. 48, p. 491.
Podlaha, E.J. and Landolt, D., J. Electrochem. Soc., 1996, vol. 143, p. 885.
Podlaha, E.J. and Landolt, D., J. Electrochem. Soc., 1997, vol. 144, p. 1672.
Younes, O. and Gileadi, E., Electrochem. Solid-State Lett. 2000, vol. 3, p. 543.
Younes, O. and Gileadi, E., J. Electrochem. Soc., 2002, vol. 149, p. C100.
Younes, O., Zhu, L., and Gilead, E., Electrochim. Acta 2003, vol. 48, p. 2551.
Obradovic, M.D., Stevanovic, R.M., and Despic, A.R., J. Electroanal. Chem., 2003, vol. 552, p. 185.
Sriraman, K.R., Raman, S.G.S., and Seshadri, S.K., Mater. Sci. Eng., A 2006, vol. 418, p. 303.
Krolikowski, A., Plonska, E., Ostrowski, A., et al., J. Solid State Electrochem., 2009, vol. 13, p. 263.
Kumar, K.A., Kalaignan, G.P., and Muralidharan, V.S., Appl. Surf. Sci. 2012, vol. 259, p. 231.
Ahmadi, M. and Guinel, M. J.-F., J. Alloys Compd., 2013, vol. 574, p. 196.
Indyka, P., Lehman, E.B., Tarkowski, L., et al., J. Alloys Compd., 2014, vol. 590, p. 75.
Zhu, L., Younes, O., Ashkenasy, N., et al., Appl. Surf. Sci. 2002, vol. 200, p. 1.
Tian, L., Xu, J., and Qiang, C., Appl. Surf. Sci. 2011, vol. 257, p. 4689.
Farzaneh, M.A., Ghavidel, M.R.Z., Raeissi, K., et al., Appl. Surf. Sci. 2011, vol. 257, p. 5919.
Jones, A.R., Hamann, J., Lund, A.C., and Schuh, C.A., Plat. Surf. Finish. 2010, vol. 97, p. 52.
Chianpairot, A., Lothongkum, G., Schuh, C.A., and Boonyongmaneert, Y., Corros. Sci. 2011, vol. 53, p. 1066.
Aljohani, T.A. and Hayden, B.E., Electrochim. Acta 2013, vol. 111, p. 930.
Anik, M. and Osseo-Asare, K., J. Electrochem. Soc., 2002, vol. 149, p. B224.
Anik, M., Turk. J. Chem., 2002, vol. 26, p. 915.
Anik, M., Corros. Sci. 2006, vol. 48, p. 4158.
Anik, M., Electrochim. Acta 2009, vol. 54, p. 3943.
Pourbaix, M., Atlas of Electrochemical Equilibria in Aqueous Solutions, Houston, TX: NACE, 1974.
Itagaki, M., Nakazawa, H., Watanabe, K., and Noda, K., Corros. Sci. 1997, vol. 39, p. 901.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Adigüzel, Z., Anik, M. Dissolution Behavior of Electrodeposited Ni–W Alloys. Prot Met Phys Chem Surf 54, 316–324 (2018). https://doi.org/10.1134/S2070205118020120
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205118020120