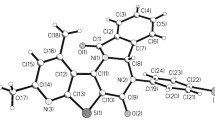
Abstract
A series of propargyl and allyl carbamates were prepared directly from propargyl and allyl alcohols and phenyl or cyclohexyl isocyanate or indirectly by generating the isocyanates in situ from the corresponding Cbz-protected amines. The obtained carbamates underwent intramolecular nucleophilic cyclization in presence of cesium hydroxide monohydrate as a base catalyst under conventional and ultrasonic irradiation conditions to give the corresponding substituted 4-(benzylidene)methylidene-2-oxazolidinones in good to excellent yields. The use of ultrasonic irradiation provided remarkably improved yield of the cyclization products and significant shortening of the reaction time. In some cases the reaction was highly stereoselective, and (Z)-4-benzylideneoxazolidinones were formed as a single stereoisomer. A series of biological tests were performed to evaluate the inhibitory potential of all synthesized compounds against some protein kinases.
Similar content being viewed by others
References
Douglas, B.J., Robert, S.L., John, J.B., Keith, E.D., Stephen, O.P., Michael, A.P., Rand, S.S., Jeffrey, A.S., and Xiao, S.T., Pestic Sci., 1999, vol. 55, p. 197.
Antonio, M.O., Jamil, C., Rubem, S.O., Alberto, L.L., Carlos, C.E., Naiara, G., and Hudson, K.T., Rev. Braz. Herb., 2015, vol. 14, p. 210.
Jo, K.A., Maheswara, M., Yoon, E., Lee, Y.Y., Yun, H., and Kang, E.J., J. Org. Chem., 2012, vol. 77, p. 2924.
Evans, D. and Gage, J., Org Synth., 1990, vol. 68, p. 83.
Kim, W.S., Yoon, E.Y., Jo, K.A., and Kang, E.J., Bull. Korean Chem. Soc., 2011, vol. 32, p. 3158.
Yewon, C., Sang, W., Anhye, K., Kyungho, J., Heesook, N., Young, L., Kyung-Sang, Y., In-Jin, J., and Jae-Yong, C., J. Antimicrob. Chemother., 2017, vol. 73, p. 183.
Kuo, H.C., Ayer, M.B., Chakravarty, P.K., Meinke, P.T., Parsons, W.H., and Tyagarajan, S., US Patent no. 7348 348 B2, 2008.
Welstead, W.J., Helsley, G.C., Taylor, C.R., Turnbull, L.B., Da Vanzo, J.P., Funderburk, W.H., and Alphin, R.S., J. Med. Chem., 1973, vol. 16, p. 1129.
Sternberg, J.A., Geffken, D., Adams, J.B., Pöstages, R., Sternberg, C.G., Campbell, C.L., and Moberg, W.K., Pest Manage. Sci., 2001, vol. 57, p. 143.
Edafiogho, I.O., Phillips, O.A., Udo, E.E., Samuel, S., and Rethish, B., Eur. J. Med. Chem., 2009, vol. 44, p. 967.
Vera-Cabrera, L., Gonzalez, E., Rendon, A., Ocampo-Candiani, J., Welsh, O., Velazquez-Moreno, V.M., Choi, S.H., and Molina-Torres, C., Antimicrob. Agents Chemother., 2006, vol. 50, p. 3170.
Naresh, A., Venkateswara Rao, M., Kotapalli, S.S., Ummanni, R., and Venkateswara Rao, B., Eur. J. Med. Chem., 2014, vol. 80, p. 295.
Ambulgekar, G.V., Samant, S.D., and Pandit, A.B., Ultrason. Sonochem., 2005, vol. 12, p. 85.
Jadhav, S.A., Sarkate, A.P., Farooqui, M., and Shinde, D.B., Synth. Commun., 2017, vol. 47, p. 1676.
Xu, H., Liao, W.M., and Li, H.F., Ultrason. Sonochem., 2007, vol. 14, p. 779.
Shachat, N. and Bagnell, J.J., J. Org. Chem., 1963, vol. 28, p. 991.
Kim, H.-K. and Lee, A., Org. Biomol. Chem., 2016, vol. 14, p. 7345.
Newton, R. and Savage, G., Aust. J. Chem., 2008, vol. 61, p. 432.
Jiang, H.-F. and Zhao, J.W., Tetrahedron Lett., 2009, vol. 50, p. 60.
Ying, M., Smentek, M.G., Ma, R., Day, C.S., Torti, S.V., and Welker, M.E., Lett. Org. Chem., 2009, vol. 6, p. 242.
Jammi, S., Mouysset, D., Siri, D., Bertrand, M.P., and Feray, L., J. Org. Chem., 2013, vol. 78, p. 1589.
Steeghs, N. and Nortier, J.W.R., Ann. Surg. Oncol., 2007, vol. 14, p. 942.
Arora, A., and Scholar, E.M., J. Pharmacol. Exp. Ther., 2005, vol. 315, p. 971.
Graczyk, P.P., J. Med. Chem., 2007, vol. 50, p. 5773.
Grega, K.C., Barbachyn, M.R., Brickner, S.J., and Mizsak, S.A., J. Org. Chem., 1995, vol 60, p. 5255.
Primot, A., Baratte, B., Gompel, M., Borgne, A., Liabeuf, S., Romette, J.L., Jho, E., Costantini, F., and Meijer, L., Protein Expression Purif., 2000, vol. 20, p. 394.
Leclerc, S., Garnier, M., Hoessel, R., Marko, D., Bibb, J.A., Snyder, G.L., Greengard, P., Biernat, J., Wu, Y.Z., and Mandelkow, E.M., J. Biol. Chem., 2001, vol. 276, p. 25.
Acknowledgements
The authors are grateful to Prof. Dr. Ramesh Ramapanicker (Indian Institute of Technology, Kanpur, India) for providing laboratory facilities.
Funding
This study was performed under financial support by the Ministry of Higher Education and Scientific Research of Algeria (grant no. 571/PNE/Doctorant/India/2016-2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
No conflict of interests is declared by the authors.
Rights and permissions
About this article
Cite this article
Ziane, S., Mazari, M.M., Safer, A.M. et al. Comparison between Conventional and Nonconventional Methods for the Synthesis of Some 2-Oxazolidinone Derivatives and Preliminary Investigation of Their Inhibitory Activity Against Certain Protein Kinases. Russ J Org Chem 55, 1061–1069 (2019). https://doi.org/10.1134/S1070428019070248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428019070248