Abstract
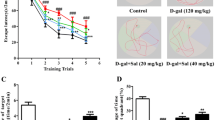
Methylene blue (MB) has a long story of use and has been employed for various diseases, however, most of its current rekindled research is related to its function in the mitochondria. MB is gaining interest as a possible treatment because mitochondrial dysfunction is an apparent unifying pathogenic characteristic of a variety of neurodegenerative conditions. Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by impairment of cognitive functions. This study aims to investigate whether MB treatment improves impaired cognitive functions and reduces hippocampal amyloid-β levels and oxidative stress in a D-galactose-induced AD mouse model. Twenty-four wild-type Balb/c mice were randomly divided into four groups (control, D-galactose-induced AD, MB-treated AD, and only MB-treated mice). Mice in the corresponding groups were injected with D-galactose (50 mg/kg, s.c.) for 60 days. In the MB treatment groups, the mice were treated with MB (2 mg/kg, orally) for the last 14 days of treatments. The Morris water maze test was used to assess spatial learning and memory functions. Amyloid β-42, malondialdehyde, superoxide dismutase, and nitric oxide levels in the hippocampi of mice were measured using ELISA and spectrophotometry. MB treatment improved impaired learning and memory functions induced by D-galactose administration and decreased amyloid β-42 concentration in the hippocampi of mice. Malondialdehyde level was found to decrease in MB-treated mice compared to D-galactose-induced AD mice in the hippocampus and plasma. The hippocampus of MB-treated mice displayed increased superoxide dismutase activity while decreased nitric oxide concentration compared to the ones of D-galactose-induced AD mice. MB has been shown to improve learning and memory impairments, as well as reduce Aβ-42 concentration and oxidative stress in the hippocampus of D-galactose-induced AD mice. The findings of this study demonstrate that MB may offer potential benefits as a repurposed agent for AD.





Similar content being viewed by others
REFERENCES
Ali, T., Badshah, H., Kim, T.H., and Kim, M.O., Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model, J. Pineal Res., 2015, vol. 58, pp. 71–85. https://doi.org/10.1111/jpi.12194
Atamna, H. and Kumar, R., Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase, J. Alzheimers Dis., 2010, vol. 20, suppl. 2, pp. S439–S452. https://doi.org/10.3233/jad-2010-100414
Atamna, H., Nguyen, A., Schultz, C., Boyle, K., Newberry, J., Kato, H., and Ames, B.N., Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways, FASEB J., 2008, vol. 22, pp. 703–712. https://doi.org/10.1096/fj.07-9610com
Berrocal, M., Corbacho, I., Gutierrez-Merino, C., and Mata, A.M., Methylene blue activates the PMCA activity and cross-interacts with amyloid beta-peptide, blocking Abeta-mediated PMCA inhibition, Neuropharmacology, 2018, vol. 139, pp. 163–172. https://doi.org/10.1016/j.neuropharm.2018.07.012
Berrocal, M., Caballero-Bermejo, M., Gutierrez-Merino, C., and Mata, A.M., Methylene blue blocks and reverses the inhibitory effect of Tau on PMCA function, Int. J. Mol. Sci., 2019, vol. 20. https://doi.org/10.3390/ijms20143521
Bruchey, A.K. and Gonzalez-Lima, F., Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue, Am. J. Pharmacol. Toxicol., 2008, vol. 3, pp. 72–79. https://doi.org/10.3844/ajptsp.2008.72.79
Boriskin, P., Gulenko, O.V., Deviatkin, A., Pavlova, O., and Toropovskiy, A., Correlation of superoxide dismutase activity distribution in serum and tissues of small experimental animals, IOP Conf. Ser.: Earth Environ. Sci., 2019, vol. 403. https://doi.org/10.1088/1755-1315/403/1/012112
Callaway, N.L., Riha, P.D., Bruchey, A.K., Munshi, Z., and Gonzalez-Lima, F., Methylene blue improves brain oxidative metabolism and memory retention in rats, Pharmacol. Biochem. Behav., 2004, vol. 77, pp. 175–181. https://doi.org/10.1016/j.pbb.2003.10.007
Cardoso, A., Magano, S., Marrana, F., and Andrade, J.P., D-galactose high-dose administration failed to induce accelerated aging changes in neurogenesis, anxiety, and spatial memory on young male Wistar rats, Rejuvenation Res., 2015, vol. 18, pp. 497–507. https://doi.org/10.1089/rej.2015.1684
Chadwick, W., Maudsley, S., Hull, W., Havolli, E., Boshoff, E., Hill, M.D.W., Goetghebeur, P.J.D., Harrison, D.C., Nizami, S., Bedford, D.C., Coope, G., Real, K., Thiemermann, C., Maycox, P., Carlton, M., and Cole, S.L., The oDGal mouse: a novel, physiologically relevant rodent model of sporadic Alzheimer’s disease, Int. J. Mol. Sci., 2023, vol. 24. https://doi.org/10.3390/ijms24086953
Chalmers, K.A. and Love, S., Neurofibrillary tangles may interfere with Smad 2/3 signaling in neurons, J. Neuropathol. Exp. Neurol., 2007, vol. 66, pp. 158–167. https://doi.org/10.1097/nen.0b013e3180303b93
Congdon, E.E., Wu, J.W., Myeku, N., Figueroa, Y.H., Herman, M., Marinec, P.S., Gestwicki, J.E., Dickey, C.A., Yu, W.H., and Duff, K.E., Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo, Autophagy, 2012, vol. 8, pp. 609–622. https://doi.org/10.4161/auto.19048
Deiana, S., Harrington, C.R., Wischik, C.M., and Riedel, G., Methylthioninium chloride reverses cognitive deficits induced by scopolamine: comparison with rivastigmine, Psychopharmacology (Berl.), 2009, vol. 202, pp. 53–65. https://doi.org/10.1007/s00213-008-1394-2
Du, X., Wang, X., and Geng, M., Alzheimer’s disease hypothesis and related therapies, Transl. Neurodegener., 2018, vol. 7, p. 2. https://doi.org/10.1186/s40035-018-0107-y
Eckert, G.P., Renner, K., Eckert, S.H., Eckmann, J., Hagl, S., Abdel-Kader, R.M., Kurz, C., Leuner, K., and Muller, W.E., Mitochondrial dysfunction—a pharmacological target in Alzheimer’s disease, Mol. Neurobiol., 2012, vol. 46, pp. 136–150. https://doi.org/10.1007/s12035-012-8271-z
Garg, G., Singh, S., Singh, A.K., and Rizvi, S.I., Antiaging effect of metformin on brain in naturally aged and accelerated senescence model of rat, Rejuvenation Res., 2017, vol. 20, pp. 173–182. https://doi.org/10.1089/rej.2016.1883
Gauthier, S., Feldman, H.H., Schneider, L.S., Wilcock, G.K., Frisoni, G.B., Hardlund, J.H., Moebius, H.J., Bentham, P., Kook, K.A., Wischik, D.J., Schelter, B.O., Davis, C.S., Staff, R.T., Bracoud, L., Shamsi, K., Storey, J.M., Harrington, C.R., and Wischik, C.M., Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial, Lancet, 2016, vol. 388, pp. 2873–2884. https://doi.org/10.1016/S0140-6736(16)31275-2
International A.S.D., World Alzheimer Report 2021, 2021.
Kazkayasi, I., Telli, G., Nemutlu, E., and Uma, S., Intranasal metformin treatment ameliorates cognitive functions via insulin signaling pathway in ICV-STZ-induced mice model of Alzheimer’s disease, Life Sci., 2022, vol. 299, p. 120538. https://doi.org/10.1016/j.lfs.2022.120538
Kumar, R., Saraswat, K., and Rizvi, S.I., 2-Deoxy-D-glucose at chronic low dose acts as a caloric restriction mimetic through a mitohormetic induction of ROS in the brain of accelerated senescence model of rat, Arch. Gerontol. Geriatr., 2020, vol. 90, p. 104133. https://doi.org/10.1016/j.archger.2020.104133
Lin, A.L., Poteet, E., Du, F., Gourav, R.C., Liu, R., Wen, Y., Bresnen, A., Huang, S., Fox, P.T., Yang, S.H., and Duong, T.Q., Methylene blue as a cerebral metabolic and hemodynamic enhancer, PLoS One, 2012, vol. 7, p. e46585. https://doi.org/10.1371/journal.pone.0046585
Medina, D.X., Caccamo, A., and Oddo, S., Methylene blue reduces aβ levels and rescues early cognitive deficit by increasing proteasome activity, Brain Pathol., 2011, vol. 21, pp. 140–149. https://doi.org/10.1111/j.1750-3639.2010.00430.x
Melis, V., Magbagbeolu, M., Rickard, J.E., Horsley, D., Davidson, K., Harrington, K.A., Goatman, K., Goatman, E.A., Deiana, S., Close, S.P., Zabke, C., Stamer, K., Dietze, S., Schwab, K., Storey, J.M., Harrington, C.R., Wischik, C.M., Theuring, F., and Riedel, G., Effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models, Behav. Pharmacol., 2015, vol. 26, pp. 353–368. https://doi.org/10.1097/fbp.0000000000000133
Mori, T., Koyama, N., Segawa, T., Maeda, M., Maruyama, N., Kinoshita, N., Hou, H., Tan, J., and Town, T., Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice, J. Biol. Chem., 2014, vol. 289, pp. 30303–30317. https://doi.org/10.1074/jbc.M114.568212
Necula, M., Breydo, L., Milton, S., Kayed, R., van der Veer, W.E., Tone, P., and Glabe, C.G., Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization, Biochemistry, 2007, vol. 46, pp. 8850–8860. https://doi.org/10.1021/bi700411k
O’Leary, J.C., 3rd, Li, Q., Marinec, P., Blair, L.J., Congdon, E.E., Johnson, A.G., Jinwal, U.K., Koren, J., 3rd, Jones, J.R., Kraft, C., Peters, M., Abisambra, J.F., Duff, K.E., Weeber, E.J., Gestwicki, J.E., and Dickey, C.A., Phenothiazine-mediated rescue of cognition in tau transgenic mice requires neuroprotection and reduced soluble tau burden, Mol. Neurodegener., 2010, vol. 5, p. 45. https://doi.org/10.1186/1750-1326-5-45
Oz, M., Lorke, D.E., Hasan, M., and Petroianu, G.A., Cellular and molecular actions of Methylene Blue in the nervous system, Med. Res. Rev., 2011, vol. 31, pp. 93–117. https://doi.org/10.1002/med.20177
Oz, M., Lorke, D.E., and Petroianu, G.A., Methylene blue and Alzheimer’s disease, Biochem. Pharmacol., 2009, vol. 78, pp. 927–932. https://doi.org/10.1016/j.bcp.2009.04.034
Paban, V., Manrique, C., Filali, M., Maunoir-Regimbal, S., Fauvelle, F., and Alescio-Lautier, B., Therapeutic and preventive effects of methylene blue on Alzheimer’s disease pathology in a transgenic mouse model, Neuropharmacology, Part A, 2014, vol. 76, pp. 68–79. https://doi.org/10.1016/j.neuropharm.2013.06.033
Parameshwaran, K., Irwin, M.H., Steliou, K., and Pinkert, C.A., D-galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice, Rejuvenation Res., 2010, vol. 13, pp. 729–735. https://doi.org/10.1089/rej.2010.1020
Peter, C., Hongwan, D., Kupfer, A., and Lauterburg, B.H., Pharmacokinetics and organ distribution of intravenous and oral methylene blue, Eur. J. Clin. Pharmacol., 2000, vol. 56, pp. 247–250. https://doi.org/10.1007/s002280000124
Rehman, S.U., Shah, S.A., Ali, T., Chung, J.I., and Kim, M.O., Anthocyanins reversed D-galactose-induced oxidative stress and neuroinflammation mediated cognitive impairment in adult rats, Mol. Neurobiol., 2017, vol. 54, pp. 255–271. https://doi.org/10.1007/s12035-015-9604-5
Schelter, B.O., Shiells, H., Baddeley, T.C., Rubino, C.M., Ganesan, H., Hammel, J., Vuksanovic, V., Staff, R.T., Murray, A.D., Bracoud, L., Riedel, G., Gauthier, S., Jia, J., Bentham, P., Kook, K., Storey, J.M.D., Harrington, C.R., and Wischik, C.M., Concentration-dependent activity of hydromethylthionine on cognitive decline and brain atrophy in mild to moderate alzheimer’s disease, J. Alzheimers Dis., 2019, vol. 72, pp. 931–946. https://doi.org/10.3233/JAD-190772
Schirmer, R.H., Adler, H., Pickhardt, M., and Mandelkow, E., “Lest we forget you—methylene blue...,” Neurobiol. Aging, 2011, vol. 32, pp. 2325.e2327–2316. https://doi.org/10.1016/j.neurobiolaging.2010.12.012
Shwe, T., Pratchayasakul, W., Chattipakorn, N., and Chattipakorn, S.C., Role of D-galactose-induced brain aging and its potential used for therapeutic interventions, Exp. Gerontol., 2018, vol. 101, pp. 13–36. https://doi.org/10.1016/j.exger.2017.10.029
Soeda, Y., Saito, M., Maeda, S., Ishida, K., Nakamura, A., Kojima, S., and Takashima, A., Methylene blue inhibits formation of tau fibrils but not of granular tau oligomers: a plausible key to understanding failure of a clinical trial for Alzheimer’s disease, J. Alzheimers Dis., 2019, vol. 68, pp. 1677–1686. https://doi.org/10.3233/jad-181001
Spires-Jones, T.L., Friedman, T., Pitstick, R., Polydoro, M., Roe, A., Carlson, G.A., and Hyman, B.T., Methylene blue does not reverse existing neurofibrillary tangle pathology in the rTg4510 mouse model of tauopathy, Neurosci. Lett., 2014, vol. 562, pp. 63–68. https://doi.org/10.1016/j.neulet.2014.01.013
Taniguchi, S., Suzuki, N., Masuda, M., Hisanaga, S., Iwatsubo, T., Goedert, M., and Hasegawa, M., Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins, J. Biol. Chem., 2005, vol. 280, pp. 7614–7623. https://doi.org/10.1074/jbc.M408714200
Wang, J., Fasina, O.B., Manzoor, M., Wang, Y., Liu, Q., Mo, J., Ohno, H., Osada, H., Xiang, L., and Qi, J., A new gentiopicroside derivative improves cognitive deficits of AD mice via activation of Wnt signaling pathway and regulation of gut microbiota homeostasis, Phytomedicine, 2023, vol. 113, p. 154730. https://doi.org/10.1016/j.phymed.2023.154730
Wei, H., Wu, G., Chen, J., Zhang, X., Xiong, C., Lei, Y., Chen, W., and Ruan, J., (2S)-5,2',5'-trihydroxy-7-methoxyflavanone, a natural product from Abacopteris penangiana, presents neuroprotective effects in vitro and in vivo, Neurochem. Res., 2013, vol. 38, pp. 1686–1694. https://doi.org/10.1007/s11064-013-1070-8
Wen, Y., Li, W., Poteet, E.C., Xie, L., Tan, C., Yan, L.J., Ju, X., Liu, R., Qian, H., Marvin, M.A., Goldberg, M.S., She, H., Mao, Z., Simpkins, J.W., and Yang, S.H., Alternative mitochondrial electron transfer as a novel strategy for neuroprotection, J. Biol. Chem., 2011, vol. 286, pp. 16504–16515. https://doi.org/10.1074/jbc.M110.208447
Wilcock, G.K., Gauthier, S., Frisoni, G.B., Jia, J., Hardlund, J.H., Moebius, H.J., Bentham, P., Kook, K.A., Schelter, B.O., Wischik, D.J., Davis, C.S., Staff, R.T., Vuksanovic, V., Ahearn, T., Bracoud, L., Shamsi, K., Marek, K., Seibyl, J., Riedel, G., Storey, J.M.D., Harrington, C.R., and Wischik, C.M., Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: cohort analysis as modified primary outcome in a phase III clinical trial, J. Alzheimers Dis., 2018, vol. 61, pp. 435–457. https://doi.org/10.3233/jad-170560
Wischik, C.M., Edwards, P.C., Lai, R.Y., Roth, M., and Harrington, C.R., Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines, Proc. Natl. Acad. Sci. U. S. A., 1996, vol. 93, pp. 11213–11218. https://doi.org/10.1073/pnas.93.20.11213
Wischik, C.M., Staff, R.T., Wischik, D.J., Bentham, P., Murray, A.D., Storey, J.M., Kook, K.A., and Harrington, C.R., Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease, J. Alzheimers Dis., 2015, vol. 44, pp. 705–720. https://doi.org/10.3233/jad-142874
Yadollahikhales, G. and Rojas, J.C., Anti-Amyloid Immunotherapies for Alzheimer’s disease: a 2023 clinical update, Neurotherapeutics, 2023. https://doi.org/10.1007/s13311-023-01405-0
Zakaria, A., Hamdi, N., and Abdel-Kader, R.M., Methylene blue improves brain mitochondrial ABAD functions and decreases Aβ in a neuroinflammatory Alzheimer’s disease mouse model, Mol. Neurobiol., 2016, vol. 53, pp. 1220–1228. https://doi.org/10.1007/s12035-014-9088-8
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The experimental procedures were approved by the Animal Experimentations Local Ethical Board of Hacettepe University (Decision no. 2021/06-13, date of ethical decision is August 24, 2021).
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kazkayasi, I., Telli, G. Methylene Blue Attenuates Impaired Cognitive Functions and Reduces Hippocampal Aβ Levels and Oxidative Stress in D-Galactose-Induced Alzheimer’s Disease Mouse Model. Biol Bull Russ Acad Sci (2024). https://doi.org/10.1134/S106235902360455X
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S106235902360455X