Abstract
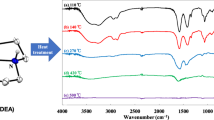
Conditions of synthesis are optimized and XRD and thermoanalytical studies are performed for normal maleate [Zn(H2O)2(C4H2O4)] and acid maleate [Zn(H2O)4(C4H3O4)2], along with acid Co(II)–Zn(II), Ni(II)–Zn(II) maleates. It is shown that when solid solutions thermally decompose in acidic maleate systems, the kinetically less stable component causes the temperature of decomposition of the more stable component to fall. It is established that the solid residue of Zn(II) maleate after heating to 500°C in a He atmosphere is a composite containing Zn oxide whose reduction to metal begins at 675°C. The decomposition of [(Co0.1Zn0.9)(H2O)4(С4H3O4)2] leads to the oozing of metallic cobalt atoms embedded in the channels of the ZnO structure that act as a catalyst for the spontaneous growth of uniform carbon nanotubes on the surface of the composite. After thermal decomposition of the samples in a system of acid Ni(II) maleate–acid Zn(II) maleate, only the bimetallic phase is observed in the composites, and there is no zinc oxide.




Similar content being viewed by others
REFERENCES
A. D. Pomogailo and V. N. Kestelman, Metallopolymer Nanocomposites (Springer, Berlin, Heidelberg, New York, 2005).
A. S. Fionov, Extended Abstract of Cand. Sci. (Chem.) Dissertation (Inst. Metall. Sci. Mater. RAS, Moscow, 2011).
Nanoparticles and Catalysis, Ed. by D. Astruc (Wiley-VCH, Weinheim, 2008).
A. D. Pomogailo and G. I. Dzhardimalieva, Metall Polymer Hybrid Nanocomposites (Nauka, Moscow, 2015) [in Russian].
Nanostructure Control of Materials, Ed. by R. H. Hannik and A. J. Hill (Woodhead, New York, 2006).
E. V. Shlyakhova, N. F. Yudanov, Yu. V. Shubin, et al., Carbon 47, 1701 (2009).
L. M. Plyasova, T. M. Yur’eva, T. A. Kriger, et al., Kinet. Katal. 36, 464 (1995).
J. Ĉernak, J. Chomiĉ, Ch. Kappenstein, and F. Robert, J. Chem. Soc., Dalton Trans., 2981 (1997).
B. P. Vesnovskii, Zh. Neorg. Khim. 21, 2651 (1976).
L. I. Yudanova, V. A. Logvinenko, N. F. Yudanov, N. A. Rudina, A. V. Ishchenko, P. P. Semyannikov, L. A. Sheludyakova, N. I. Alferova, A. I. Romanenko, and O. B. Anikeeva, Inorg. Mater. 49, 1055 (2013).
L. I. Yudanova, V. A. Logvinenko, L. A. Sheludyakova, N. F. Yudanov, P. P. Semyannikov, S. I. Kozhemya-chenko, I. V. Korol’kov, N. A. Rudina, and A. V. Ishchen-ko, Russ. J. Inorg. Chem. 59, 1180 (2014).
A. S. Antsyshkina, L. M. Dikareva, M. A. Porai-Koshits, et al., Koord. Khim. 8, 1256 (1982).
A. Sequoia, H. Rajagopal, M. P. Gupta, et al., Acta Crystallogr. 48, 1192 (1992).
J. Ĉernak, D. Mikloŝ, C. Kappenstein, et al., Chem. Papers 54, 282 (2000).
A. D. Pomogailo, A. S. Rozenberg, and I. E. Uflyand, Nanoparticles of Metals in Polymers (Khimiya, Moscow, 2000) [in Russian].
T. Penkala, Essays on Crystal Chemistry (Khimiya, Leningrad, 1974) [in Russian].
S. Abrahams and J. Bernstein, Acta Crystallogr., B 25, 1233 (1969).
V. M. Goldschmidt, Kristallchemie (von Gustav Fisher, Jena, 1934).
State Diagrams of Binary Metal Systems, The Handbook, Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 2001), Vol. 3, Part 1, p. 669 [in Russian].
G. B. Bokii, Crystal Chemistry (Nauka, Moscow, 1971), p. 258 [in Russian].
R. B. Vasil’ev and D. N. Dirin, Quantum Dots: Synthesis, Properties, Application (Mosk. Gos. Univ., Moscow, 2007) [in Russian].
ACKNOWLEDGMENTS
The authors are grateful to L.A. Sheludyakova for her valuable comments and advice during the writing of this work; and to N.F. Beizel’, A.P. Zubareva, and O.S. Koshcheeva for performing our elemental analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Polyakov
Rights and permissions
About this article
Cite this article
Yudanova, L.I., Logvinenko, V.A., Korol’kov, I.V. et al. Thermal Decomposition in Systems of Acid Zn(II), Co(II), and Ni(II) Maleates with the Formation of Metallic Nanoparticles. Russ. J. Phys. Chem. 92, 2247–2252 (2018). https://doi.org/10.1134/S003602441811047X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602441811047X