Abstract
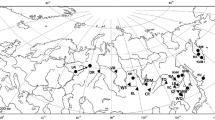
Nucleotide sequences of the NADH dehydrogenase subunit 2 gene (ND2) of mitochondrial DNA (mtDNA) were determined in stoats (Mustela ermine) from northeastern Kamchatka. Analysis of the ND2 variability in stoats, which is represented here and, also, in previous studies, shows that the divergence level between American and Eurasian mtDNA haplotypes is about 5%, whereas that among Eurasian ones is as low as 0.5%. The results of the phylogenetic analysis also indicate a highly significant differentiation between the American and Eurasian mtDNA lineages, whereas a single Kamchatkan cluster of mtDNA haplotypes is recognized in the Eurasian mtDNA clade with high confidence. Molecular dating shows that separation of the ancestral stoat population occurred approximately 1.3–1.6 million years ago, but the Eurasian mtDNA lineages diverged about 300 ka ago. The evolutionary age of Kamchatkan mtDNA haplotypes is about 95–120 ka, which contradicts other authors’ suggestions about the last postglacial (19–26.5 ka) recolonization of Eurasia by stoat populations. This inconsistency is discussed.
Similar content being viewed by others
References
Abramson, N.I., Phylogeography: results, problems, and prospects, Inform. Vestn. VOGi, vol. 11, no. 2, pp. 307–331.
Avise, J.C., Genetrees and organismal histories: a phylogenetic approach to the population biology, Evolution, 1989, vol. 43, pp. 1192–1208.
Clark, P.U., Dyke, A.S., Shakun, J.D., et al., Thelastglacial maximum, Science, 2009, vol. 325, pp. 710–714.
Delisle, I. and Strobeck, C., A phylogeny of the Caniformia (order Carnivora) based on 12 complete protein-coding mitochondrial genes, Mol. Phylogenet. Evol., 2005, vol. 37, pp. 192–201.
Domingo-Roura, X., Lopez-Giraldez, F., Saeki, M., and Marmi, J., Phylogenetic inference and comparative evolution of a complex microsatellite and its flanking regions in carnivores, Genet. Res., 2005, vol. 85, pp. 223–233.
Drummond, A.J. Suchard, M.A., Xie, D., et al., Bayesian phylogenetics with BEAUti and the BEAST1.7, Mol. Biol. Evol., 2012, vol. 29, pp. 1969–1973.
Finnila, S., Lehtonen, M.S., and Majamaa, K., Phylogenetic network for European mtDNA, Am. J. Hum. Genet., 2001, vol. 68, pp. 1475–1484.
Fleming, M.A. and Cook, J.A., Phylogeography of endemic ermine (Mustela erminea) in southeast Alaska, Mol. Ecol., 2002, vol. 11, pp. 795–807.
Flynn, J.J., Finarelli, J.A., Zehr, S., et al., Molecular phylogeny of the Carnivora (Mammalia): assessing the impact of increased sampling on resolving enigmatic relationships, Syst. Biol., 2005, vol. 54, pp. 317–337.
Fulton, T.L. and Strobeck, C., Novel phylogeny of the raccoon family (Procyonidae: Carnivora) based on nuclear and mitochondrial DNA evidence, Mol. Phylogenet. Evol., 2007, vol. 43, pp. 1171–1177.
Grafodatskii, A.C., Volobuev, V.T., Ternovskii, D.V., and Radzhabli, S.I., G-banding of chromosomes of seven species of Mustelidae (Carnivora, Mustelidae), Zool. Zh., 1976, vol. 55, no. 11, pp. 1704–1709.
Harding, L.E. and Smith, F.A., Mustela or Vison? Evidence for the taxonomic status of the American mink and a distinct biogeographic radiation of American weasels, Mol. Phylogenet. Evol., 2009, vol. 52, pp. 632–642.
Hosoda, T., Satoj, J., Shimada, K.L., et al., Independent nonframeshift deletions in the MC1R gene are not associated with melanistic coat coloration in three mustelid lineages, J. Hered., 2005, vol. 96, pp. 607–613.
Hosoda, T., Sato, J.J., Lin, L.-K., et al., Phylogenetic history of mustelid fauna in Taiwan inferred from mitochondrial genetic loci, Can. J. Zool., 2011, vol. 89, pp. 559–569.
Irwin, D.E., Phylogeographic breaks without geographic barriers to gene flow, Evolution, 2002, vol. 56, pp. 2383–2394.
King, C.M., Mustela erminea, Mammal Sp., 1983, vol. 195, pp. 1–8.
Koepfli, K.-P. and Wayner, K., Phylogenetic relationships of otters (Carnivora: Mustelidae) based on mitochondrial cytochrome b sequences, J. Zool., 1998, vol. 246, pp. 401–416.
Koepfli, K.-P., Deerek, A., Slater, G.J., et al., Multigene phylogeny of the Mustelidae: resolving relationships, tempo and biogeographic history of a mammalian adaptive radiation, BMC Biol., 2008, vol. 6, p. 10.
Kurose, N., Abramov, A.V., and Masuda, R., Comparative phylogeography between the ermine Mustela ermine and the least weasel M. nivalis of Palaearctic and Nearctic regions, based on analysis of mitochondrial DNA control region sequences, Zool. Sci., 2005, vol. 22, pp. 1069–1078.
Kurten, B. and Anderson, E., Pleistocene Mammals of North America, New York: Columbia Univ. Press, 1980.
Li, B., Malyarchuk, B., Ma, Z., et al., Phylogeography of sable (Martes zibellina L. 1758) in the southeast portion of its range based on mitochondrial DNA variation: highlighting the evolutionary history of the sable, Acta Theriol., 2013, vol. 58, pp. 139–148.
Lushnikova, T.P., Omelyanchuk, L.V., and Grafodatskii, A.C., Phylogenetic relations of closely related species of Mustelidae. Interspecies variability of localization of restriction sites of BamHI-repeats, Genetika, 1989, vol. 25, no. 6, pp. 1089–1094.
Malyarchuk, B.A., Rogozin, I.B., Berikov, V.B., and Derenko, M.V., Analysis of phylogenetically reconstructed mutational spectra in human mitochondrial DNA control region, Hum. Genet., 2002, vol. 111, pp. 46–53.
Malyarchuk, B.A., Adaptive intraspecific divergence: an example using the animal cytochrome b gene, Russ. J. Genet., 2011, vol. 47, no. 8, pp. 979–986.
Martinkova, N., McDonald, R.A., and Searle, J.B., Stoats (Mustela erminea) provide evidence of natural overland colonization of Ireland, Proc. R. Soc. B, 2007, vol. 274, pp. 1387–1393.
Pavlinov, I.Ya., Priroda Rossii: zhizn’ zhivotnykh. Mlekopitayushchie (chast’ 1) (Nature of Russia: The Lives of Animals. Mammals (Part 1)), Moscow: AST, 1999.
Rogozin, I.B., Glazko, V.I., and Kunin, E.V., The molecular basis of the law of homological rows of variability by N.I. Vavilov, Inform. Vestn. VOGiS, 2008, vol. 12, no. 3, pp. 362–371.
Rozhnov, V.V., Meshchersky, I.G., Pishchulina, S.L., et al., Genetic analysis of sable (Martes zibellina) and pine marten (M. martes) populations in sympatric part of distribution area in the Northern Urals, Russ. J. Genet., 2010, vol. 46, no. 4, pp. 488–492.
Rozhnov, V.V., Pishchulina, S.L., Meshchersky, I.G., et al., Genetic structure of sable (Martes zibellina L.) in Eurasia—analysis of the mitochondrial lineages distribution, Russ. J. Genet., 2013, vol. 49, no. 2, pp. 220–227.
Sato, J.J., Yasuda, S.P., and Hosoda, T., Genetic diversity of the Japanese marten (Martes melampus) and its implications for the conservation unit, Zool. Sci., 2009, vol. 26, pp. 457–466.
Sato, J.J., Hosoda, T., Kryukov, A.P., et al., Genetic diversity of the sable (Martes zibellina, Mustelidae) in Russian Far East and Hokkaido inferred from mitochondrial NADH dehydrogenase subunit 2 gene sequences, Mamm. Stud., 2011, vol. 36, pp. 209–222.
Tamura, K., Peterson, D., Peterson, N., et al., MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods, Mol. Biol. Evol., 2011, vol. 28, pp. 2731–2739.
Xu, C.Z., Zhang, H.H., Ma, J.Z., et al., The complete mitochondrial genomeofsable, Martes zibellina, Mitochondrial DNA, 2012. vol. 23, pp. 167–169
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © B.A. Malyarchuk, G.A. Denisova, M.V. Derenko, 2014, published in Vavilovskii Zhurnal Genetiki i Selektsii, 2014, Vol. 18, No. 3, pp. 456–462.
Rights and permissions
About this article
Cite this article
Malyarchuk, B.A., Denisova, G.A. & Derenko, M.V. Molecular dating of intraspecific differentiation of stoats (Mustela erminea) based on the variability of the mitochondrial ND2 gene. Russ J Genet Appl Res 5, 16–20 (2015). https://doi.org/10.1134/S2079059715010074
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079059715010074