Abstract
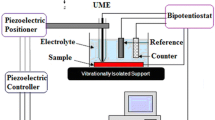
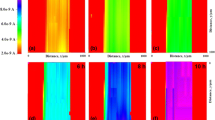
The effect of introducing chromium oxide (Cr2O3) nanoparticles in the epoxy coatings on carbon steel was analyzed using Scanning Electrochemical Microscopy (SECM) and Electrochemical Impedance Spectroscopy (EIS). Localized measurements such as oxygen consumption and iron dissolution were observed using SECM in 3.5% NaCl in the epoxy coated sample. Line profile and topographic image analysis were measured by applying –0.70 and +0.60 V as the tip potential for the cathodic and anodic reactions, respectively. The tip current at –0.70 V for the epoxy coated sample with Cr2O3 nanoparticles decreased rapidly, which is due to cathodic reduction of dissolved oxygen. The EIS measurements were conducted in 3.5% NaCl after wet and dry cyclic corrosion test. The increase in the film resistance (Rf) and charge transfer resistance (Rct) values was confirmed by the addition of Cr2O3 nanoparticles in the epoxy coating. SEM/EDX analysis showed that Cr2O3 was enriched in corrosion products at a scratched area of the coated steel after corrosion testing. FIB-TEM analysis confirmed the presence of the nanoscale oxide layer of Cr2O3 in the rust of the steel, which had a beneficial effect on the corrosion resistance of coated steel by forming protective corrosion products in the wet/dry cyclic test.













Similar content being viewed by others
REFERENCES
Bardel, E., Corrosion and Protection, London: Springer, 2003.
Roberge, P.R., Handbook of Corrosion Engineering, New York: McGraw-Hill, 1999.
Ferreira, E.S., Giacomelli, C., Giacomelli, F.C., and Spinelli, A., Mater. Chem. Phys., 2004, vol. 83, pp. 129–134.
Popovic, M.M., Grgur, B.N., and Miskovic-Stankovic, V.B., Prog. Org. Coat., 2005, vol. 52, pp. 359–365.
Leidheiser, H., Jr., Corrosion, 1982, vol. 38, pp. 374–383.
González-García, Y., González, S., and Souto, R.M., Corros. Sci., 2007, vol. 49, pp. 3514–3526.
Walter, G.W., Corros. Sci., 1986, vol. 26, pp. 27–38.
van Westing, E.P.M., Ferrari, G.M., and de Wit, J.H.W., Corros. Sci., 1993, vol. 34, pp. 1511–1530.
Rashvand, M. and Ranjbar, Z., Prog. Org. Coat., 2013, vol. 76, pp. 1413–1417.
Mills, D.J., Jamali, S.S., and Paprocka, K., Surf. Coat. Technol., 2012, vol. 209, pp. 137–142.
Fushimi, K. and Seo, M., Electrochim. Acta, 2001, vol. 47, pp. 121–127.
Seegmiller, J.C. and Buttry, D.A., J. Electrochem. Soc., 2003, vol. 150, pp. B413–B418.
Izquierdo, J., Santana, J.J., González, S., and Souto, R.M., Electrochim. Acta, 2010, vol. 55, pp. 8791–8800.
Martin, F.J., Cheek, G.T., O’Grady, W.E., and Natishan, P.M., Corros. Sci., 2005, vol. 47, pp. 3187–3201.
Souto, R.M., Gonzalez-Garcıa, Y., Gonzalez, S., and Burstein, G.T., Corros. Sci., 2004, vol. 46, pp. 2621–2628.
Souto, R.M., González-García, Y., Izquierdo, J., and González, S., Corros. Sci., 2010, vol. 52, pp. 748–753.
Akid, R. and Mills, D.J., Corros. Sci., 2001, vol. 43, pp. 1203–1216.
Raj, X.Joseph and Nishimura, T., Prot. Met. Phys. Chem. Surf., 2016, vol. 52, pp. 543–554.
Raj, X.Joseph and Nishimura, T., J. Mater. Eng. Perform., 2016, vol. 25, pp. 474–486.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
X. Joseph Raj Investigation into the Effect of Cr2O3 Nanoparticles on the Protective Properties of Epoxy Coatings on Carbon Steel in 3.5% NaCl Solution by Scanning Electrochemical Microscopy. Prot Met Phys Chem Surf 55, 80–88 (2019). https://doi.org/10.1134/S2070205119010167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2070205119010167