Abstract—
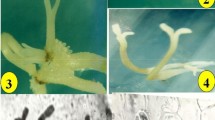
The morphogenetic potential of in vitro culture of the valuable medicinal plant Rhodiola rosea from six natural habitats was evaluated as a basis for developing effective methods for the reproduction and conservation of the rare species. The influence of different quality seeds from different habitats on germination in vitro, the dependence of the regenerative capacity of R. rosea shoots on the concentration and combination of 6-benzylaminopurine (BAP) and α-naphthylacetic acid (NAA), and the effect of shoot precultivation on media with growth regulators on the development parameters of regenerants on a hormone-free medium 1/2 MS were studied. The dependence of in vitro germination of R. rosea seeds on the habitat of samples and shelf life is shown. It was noted that the introduction of growth regulators into the nutrient medium led to an increase in the multiplication factor by 1.9–2.8 times and a decrease in the height of the shoots by 2.4–3.3 times. Variant no. 2 from Sakhalin oblast was characterized by the highest average shoot height and breeding rate. For variant No. 4 from Kamchatka krai, various morphogenic reactions (sprouting, callus formation, and flowering of plants) have been noted in an in vitro culture. For all studied variants, 100% rooting on a 1/2 MS medium is typical. For variants nos. 1 and 5, the positive effect of precultivation of explants on media containing 1 mg/L BAP alone or in combination with 1 mg/L NAA is shown to obtain optimal indicators of rhizogenesis and the development of regenerants. Significant differences in the parameters of growth and development of explants in in vitro culture depending on the composition of the nutrient medium and habitat of R. rosea are shown.




Similar content being viewed by others
REFERENCES
Ahmed, F., Filion, V., Saleem, A., and Arnason, J.T., Phytochemistry of Rhodiola rosea, in Rhodiola rosea, Cuerrier, A. and Ampong-Nyarko, K., Eds., Boca Raton, FL: CRC Press, 2014, pp. 65–87.
Al-Khateeb, A.A., The problems facing the use of tissue culture technique in date palm (Phoenix dactylifera L.), Sci. J. King Faisal Univ., 2008, vol. 9, pp. 85–104.
Bae, K.H., Ko, M.S., Kim, N.Y., Song, J.M., and Song, G.P., In vitro propagation and multiple shoot induction of Rhodiola rosea L. by axillary bud culture, J. Plant Biotechnol., 2012, vol. 39, pp. 114–120.
Erst, A.A., Gorbunov, A.B., and Erst, A.S., Effect of concentration, method of auxin application and cultivation conditions on in vitro rooting of bog blueberry (Vaccinium uliginosum L.), J. Berry Res., 2018, vol. 8, no. 1, pp. 41–53.
Furmanowa, M., Oledzka, H., Michalska, M., Sokolnicka, I., and Radomska, D., Rhodiola rosea L. (roseroot): In vitro regeneration and the biological activity of roots, in Medicinal and Aromatic Plants VIII, Biotechnology in Agriculture and Forestry Series vol. 33, Bajaj, Y.P.S., Ed., New York: Springer-Verlag, 1995, pp. 412–426.
Heylen, C. and Vendrig, J.C., The influence of different cytokinins and auxins on flower neoformation in thin cell layers of Nicotiana tabacum L., Plant Cell Physiol., 1988, vol. 29, pp. 665–671.
Ishmuratova, M.M., Clonal micropropagation of Rhodiola rosea L. and R. iremelica Boriss. in vitro, Rastit. Resur., 1998, vol. 34, no. 1, pp. 12–23.
Ishmuratova, M.M. and Satsyperova, I.F., Initial stages of ontogenesis and some biological features of development of Rhodiola rosea L. and R. iremeloca Boriss. introduced in Bashkiria, Rastit. Resur., 1998, vol. 34, no. 1, pp. 3–11.
Kim, E.F., Rhodiola rosea (golden root) fam. Crassulaceae and biological principles of culturing, Extended Abstract of Doctoral (Biol.) Dissertation, Novosibirsk, 1999.
Krasnaya kniga Rossiiskoi Federatsii (rasteniya i griby) (The Red Data Book of Russian Federation: Plants and Fungi), Moscow: KMK, 2008.
Liu, H.-J., Guo, D., Yan, Q., Liu, Y.J., and Liu, C.Z., Tissue culture of four Rhodiola species, Acta Bot. Boreali-Occident. Sin., 2006, no. 10, pp. 207–210.
Muraseva, D.S., Novikova, T.I., and Erst, A.A., In vitro propagation and conservation of rare species Fritillaria meleagris L. from floral explants, Contemp. Probl. Ecol., 2015, vol. 8, no. 6, pp. 754–763.
Murashige, T. and Skoog, F., A revised medium for rapid growth and bioassays with tobacco tissue cultures, Physiol. Plant, 1962, vol. 15, no. 13, pp. 473–497.
Nakhooda, M., Watt, M. P., and Mycock, D., Auxin stability and accumulation during in vitro shoot morphogenesis influences subsequent root induction and development in Eucalyptus grandis, Plant Growth Regul., 2011, vol. 65, pp. 263–271.
Tasheva, K. and Kosturkova, G., Bulgarian golden root in vitro cultures for micropropagation and reintroduction, Central Eur. J. Biol., 2010, vol. 5, no. 6, pp. 853–863.
Tasheva, K. and Kosturkova, G., The role of biotechnology for conservation and biologically active substances production of Rhodiola rosea: endangered medicinal species, Sci. World J., 2012, vol. 2012, art. ID 274942.
Tkachenko, K.G., Heterodiasporia and seasonal fluctuations in germination rhythms, Nauchn. Ved. Belgorod. Gos. Univ., 2009, no. 11 (66), pp. 44–50.
Yan, M.M., Xu, C., Kim, C.H., Um, Y.C., Bah, A.A., and Guo, D.P., Effects of explant type, culture media and growth regulators on callus induction and plant regeneration of Chinese jiaotou (Allium chinense), Sci. Hortic., 2009, vol. 123, no. 1, pp. 124–128.
Yin, W.B., Li, W., Du, G.S., and Huang, Q.N. Studies on tissue culture of Tibetan Rhodiola rosea, Acta Bot. Boreali-Occident. Sin., 2004, vol. 24, pp. 1506–1510.
Zhang, T., In vitro flowering of Perilla frutescens, In Vitro Cell. Dev. Biol.: Plant, 2007, vol. 43, pp. 91–94.
ACKNOWLEDGEMENTS
In preparing the publication, materials from the bioresource scientific collection of the CSBG SB RAS “Collections of Living Plants in Open and Closed Ground,” USU no. USU 440534, were used. Part of the work was performed on equipment from CCU “Microscopic Analysis of Biological Objects” at CSBG SB RAS.
Funding
This work was performed as part of the state assignment of the Central Siberian Botanical Garden, Siberian Branch, Russian Academy of Sciences, under the project “Assessing the Morphogenetic Potential of Plant Populations in North Asia by Experimental Methods” no. АААА-А17-117012610051-5.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest.
Statement of the welfare of animals. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Shulskaya
Rights and permissions
About this article
Cite this article
Erst, A.A., Yakubov, V.V. Regenerative In Vitro Capacity of Rare Species Rhodiola rosea L. from Various Habitats. Contemp. Probl. Ecol. 12, 368–376 (2019). https://doi.org/10.1134/S1995425519040036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1995425519040036