Abstract
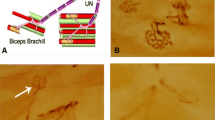
Using laser confocal microscopy and some vital fluorescent dyes (acridine orange, RH 414, DiOC6(3), rhodamine 123, fluorescein dextran), changes of the T-system and cellular acidic organelles were studied during spreading (Zenker’s) necrosis of isolated frog skeletal muscle fibers. The most characteristic of the initial stages of development of Zenker’s necrosis is the formation of numerous vacuoles as a result of local T-system swellings. The vacuole length can reach tens of micrometers. They are located both near nuclear poles and between myofibrils. Until the moment of contraction knot separation, the vacuoles preserve their connections with normal T-tubules and under certain conditions (glycerol influx to the fiber) are reversible. The vacuoles deform nuclei and cisternae of the sarcoplasmic reticulum. Acidic cell organelles accumulating acridine orange (lysosomes, late endosomes, trans-Golgi cisternae) are located in the immediate vicinity both of normal and of vacuolated T-tubules. In the course of the development of the pathological process, the size and number of acidic organelles increases and they tend to be clustered. Vacuolation of the T-system during necrosis was not accompanied by vacuole content acidification. At late stages of necrosis, alterations of nuclei and sarcoplasmic reticulum were observed. The role of cellular acidic organelles and of the T-system vacuolation in development of various muscle pathologies is discussed.
Similar content being viewed by others
Abbreviations
- AO:
-
acridine orange
- SR:
-
sarcoplasmic reticulum
- ZN:
-
Zenker’s necrosis
References
Bechet, D., Tassa, A., Taillandier, D., Combaret, L., and Attaix, D., Lysosomal Proteolysis in Skeletal Muscle, Int. J. Biochem. Cell Biol., 2005, vol. 37, pp. 2098–2114.
Bird, J.W.C., Roisen, F.J., Yorke, G., Lee, J.A., McElligott, M.A., Triemer, D.F., and John A.S., Lysosomes and Proteolytic Enzyme Activities in Cultured Striated Muscle Cells, J. Histochem. Cytochem., 1981, vol. 29, pp. 431–439.
Carpenter, S. and Karpati, G., Segmental Necrosis and Its Demarcations in Experimental Micropuncture Injury of Skeletal Muscle Fibers., J. Neurobiol. Exp. Neurology, 1989, vol. 48, pp. 154–170.
Casademont, J., Carpenter, S., and Karpati, G., Vacuolation of Muscle Fibers near Sarolemmal Breaks Represents T-Tubule Dilatation Secondary to Enhanced Sodium Pump Activity, J. Neurobiol. Exp. Neurology, 1988, vol. 47, pp. 619–629.
Clerc, S. and Barenholz, Y., A Quantitative Model for Using Acridine Orange as a Transmembrane pH Gradient Probe, Analyt. Biochem., 1998, vol. 259, pp. 104–111.
Corbett A.J., and Pollock, M., Experimental Potassium Depletion Myopathy, J. Neurol. Sci., 1981, vol. 49, pp. 193–206.
Dexxx Bleecker, J.L., Engel, A.G., and Winkelmann, J.C., Localization of Dystrophin and β-Spectrin in Vacuolar Myopathies, Am. J. Pathol., 1993, vol. 143, pp. 1200–1208.
Duncan, C.J., Role of Calcium in Triggering Rapid Ultrastructural Damage in Muscle: a Study with Chemically Skinned Fibers, J. Cell Sci., 1987, vol. 87, pp. 581–594.
Engel, A.G., Evolution and Content of Vacuoles in Primary Hypokalemic Periodic Paralysis, Mayo Clin. Proc., 1970, vol. 45, pp. 774–813.
Gamaley, I.A., and Kaulin, A.B., The vital Study of Hydrophobic Interactions in Protein Fibrils by Means of Polarizational-fluorescent Method. II. Muscle Fibers, Tsitologiya, 1988, vol. 30, no. 1, pp. 49–50.
Haugland R.P., Handbook of Fluorescent Probes and Research Chemicals, Molecular Probes, Inc., Ninth Edition, Eugene, OR, USA, 2004.
Kalamkarova, M.B., Kofman, E.B., Filatova, L.G., and Shtrankfeld, I.G., On Binding of Acridine Orange with Muscle Proteins, Tsitologiya, 1965, vol. 7, no. 2, pp. 240–243.
Kao, M.D. and Gordon, A.M., Alteration of Skeletal Muscle Cellular Structures by Potassium Depletion, Neurology, 1977, vol. 27, pp. 855–860.
Karpati, G. and Carpenter, S., Micropuncture Lesions of Skeletal Muscle Cells: a New Experimental Model for the Study of Muscle Cell Damage, Repair, and Regeneration, Disorders of the Motor Unit, New York: John Wiley, 1982, pp. 517–533.
Krolenko, S.A., Amos, W.B., and Lucy, J.A., Reversible Vacuolation of the Transverse Tubules of Frog Skeletal Muscle: a Confocal Fluorescence Microscopy Study, J. Muscle Res. Cell Motil., 1995, vol. 16, pp. 401–411.
Krolenko, S., Adamyan, S., Belyaeva, T., and Mozhenok, T., Acridine Orange Accumulation in Acid Organelles of Normal and Vacuolated Frog Skeletal Muscle Fibers, Cell Biol. Int., 2006, vol. 30, pp. 933–939.
Krolenko, S.A., Sistema myshechnykh volokon (The T-System of Muscle Fibers), Leningrad: Nauka, 1975.
Krolenko, S.A., Adamyan, S.Ya., Belyaeva, T.N., and Mozhenok, T.P., Localization of Acidic Organelles in Frog Skeletal Muscle Fibers, Tsitologiya, 2003, vol. 45, no. 7, pp. 714–721.
Krolenko, S.A., Amos, W.B., Brown, S.C., Tarunina, M.V., and Lucy, J.A., Accessibility of T-Tubule Vacuoles to Extracellular Dextran and DNA: Mechanism and Potential Application of Vacuolation, J. Muscle Res. Cell Motil., 1998, vol. 19, pp. 603–611.
Krolenko, S.A. and Adamyan, S.Ya., Stereological Analysis of the System Vacuolation in Frog Muscle Fiber T-System Revealed by Confocal Fluorescence Microscopy, Tsitologiya, 2000, vol. 42, no. 12, pp. 1125–1133.
Krolenko, S.A. and Lucy, J.A., Reversible Vacuolation of T-Tubules in Skeletal Muscle: Mechanisms and Implications for Cell Biology, 2001, Int. Rev. Cytol., 2001, vol. 202, pp. 243–298.
Krolenko, S.A. and Lucy, J.A., Vacuolation in T-Tubules as a Model for Tubular-vesicular Transformation in Biomembrane System, Cell Biol. Int., 2002, vol. 26, pp. 893–904.
Libelius, R., Jirmanova, I., Lundquist, I., Thesleff, S., and Barnard, E.A., T-Tubule Endocytosis in Dystrophic Chicken Muscle and its Relation to Muscle Fiber Degeneration, Acta Neuropathol., 1979a, vol. 48, pp. 31–38.
Libelius, R., Josefsson, J.-O., and Lundquist, I., Endocytosis in Chronically Denervated Mouse Skeletal Muscle. A Biochemical and Ultrastructural Study with Horseradish Peroxidase, J. Neurosci., 1979b, vol. 4, pp. 283–292.
Lu, Z., Joseph, D., Bugnard, E., Zaal, K.J., and Ralston, E., Golgi Complex Reorganization during Muscle Differentiation: Visualization in Living cells and Mechanism, Mol. Biol. Cell, 2001, vol. 12, pp. 795–808.
Nonaka, I., and Sugita, H., Intracytoplasmic Vacuoles in αW Fibers of Dystrophic Chicken Muscle—Probable Early Pathologic Event Initiates Massive Fiber Necrosis, Acta Neuropathol., 1981, vol. 55, pp. 173–181.
Palmgren, M.G., Acridine Orange as a Probe for Measuring pH Gradients across Membranes: Mechanism and Limitations, Analyt. Biochem., 1991, vol. 192, pp. 316–321.
Ralston, E., Changes in Architecture of the Golgi Complex and Other Subcellular Organelles during Myogenesis, J. Cell Biol., 1993, vol. 120, pp. 399–409.
Ralston, E., Ploug, T., Kalhovde, J., and Lomo, T., Golgi Complex, Endoplasmic Reticulum Exit Sites, and Microtubules in Skeletal Muscle Fibers are Organized by Patterned Activity, J. Neurosci., 2001, vol. 21, pp. 875–883.
Schindler, M., Grabski, S., Hoff, E., and Simon, S.M., Defective pH Regulation in Acidic Compartments in Human Breast Cancer Cells (MCF-7) Is Normalized in Adriamycin-resistant Cells (MCF-7adr), Biochemistry, 1996, vol. 35, pp. 2811–2817.
Voigt, T., and Dauber, W., About the T-system in the Myofibril-free Sarcoplasm of the Frog Muscle Fiber, Tissue and Cell., 2004, vol. 36, pp. 245–248.
Vult von Steyern, F., Josefsson, J-O., and Tågerud, S., Rhodamin B, a Fluorescent-probe for Acid Organelles in Denervated Skeletal Muscle, J. Histochem. Cytochem., 1996, vol. 44, pp. 267–274.
Weisz, O.A., Acidification and Protein Traffic, Int. Rev. Cytol., 2003, vol. 226, pp. 259–319.
Zelenin, A.V., Acridine Orange as a Probe for Cell and Molecular Biology, Fluorescent and Luminescent Probes for Biological Activity, London: Academic Press, 1999, pp. 117–135.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.A. Krolenko, S.Ya. Adamyan, T.N. Belyaeva, T.P. Mozhenok, A.V. Salova, 2007, published in Tsitologiya, Vol. 49, No. 2, 2007.
Rights and permissions
About this article
Cite this article
Krolenko, S.A., Adamyan, S.Y., Belyaeva, T.N. et al. Confocal microscopy study of membrane organelles of the skeletal muscle fiber in the process of Zenker’s (spreading) necrosis. Cell Tiss. Biol. 1, 183–190 (2007). https://doi.org/10.1134/S1990519X07020101
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1990519X07020101