Abstract
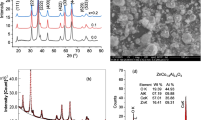
Effect of synthesis conditions on the structure and optical properties of hybrid copolymers based on Ti(OPri)4 and hydroxyethyl methacrylate was determined. Raising the concentration of the methacrylic monomer in the system leads to a longer hydrolytic polycondensation of titanium alkoxide and faster radical polymerization of the organic monomer. Copolymers containing poly(titanium oxide) with a nearly anatase structure were obtained in the conditions of a double-stage synthesis including successive stages of low-temperature hydrolytic polycondensation and polymerization. In the case of a single-stage synthesis at 70°C, which combines simultaneously occurring polycondensation and polymerization processes, the copolymer contains the anatase (75%) and rutile (25%) forms of poly(titanium oxide).
Similar content being viewed by others
References
Pomogailo, A.D., Rozenberg, A.S., and Uflyand, I.E., Nanochastitsy metallov v polimerakh (Metal Nanoparticles in Polymers), Moscow: Khimiya, 2000.
Srisitthiratkul, Ch., Pongsorrarith V., and Intasanta, N., Appl. Surf. Sci., 2011, vol. 257, no. 21, pp. 8850–8856.
Wang, X., Hou, X., Luan, W., et al., Appl. Surf. Sci., 2012, vol. 258, no. 20, pp. 8241–8246.
Kubacka, A., Ferrer, M., Fernandez-Garcia, M., et al., Appl. Catal. B, 2011, vol. 104, nos. 3–4, pp. 346–352.
Ravirajan, P., Bradley, D., Nelson, J., et al., Appl. Phys. Lett., 2005, vol. 86, no. 14, pp. 143101.
Zeng, T.-W., Lo, H.-H., Chang, Ch.-H., et al., Sol. Energy Mater. Sol. Cells, 2009, vol. 93, nos. 6–7, pp. 952–957.
Huisman, C.L., Schoonman, J., and Goossens, A., Sol. Energy Mater. Sol. Cells, 2005, vol. 85, no. 115, pp. 115–124.
Museur, L., Gorbovyi, P., Traore, M., et al., J. Lumin., 2012, vol. 132, no. 5, pp. 1192–1199.
Liang, Y., Dvornikov, A.S., and Rendzepis, P.M., Opt. Comm., 2003, vol. 223, nos. 1–3, pp. 61–66.
Reyes-Esqueda, J.-A., Vebreb, L., Lecaque, R., et al., Opt. Comm., 2003, vol. 220, nos. 1–3, pp. 59–66.
Sarantopoulos, Ch., Puzenat, E., Guillard, Ch., et al., Appl. Catal. B, 2009, vol. 91, nos. 1–2, pp. 225–233.
Huang, X., Yuan, J., Shi, J., et al., J. Hazard. Mater., 2009, vol. 171, nos. 1–3, pp. 827–832.
Ao, C.H., Lee, S.C., and Yu, J.C., J. Photochem. Photobiol., A, 2003, vol. 156, nos. 1–3, pp. 171–177.
Nakata, K. and Fujishimaa, A., J. Photochem. Photobiol., C, 2012, vol. 13, no. 3, pp. 169–189.
Sanchez, C., Soler-Illia, G.J., Ribot, F., et al., Chem. Mater., 2001, vol. 13, no. 10, pp. 3061–3083.
Rozes, L. and Sanchez, C., Chem. Soc. Rev., 2011, vol. 40, no. 2, pp. 1006–1030.
Kallala, M., Sanchez, C., and Cabane, B., Phys. Rev. E, 1993, vol. 48, no. 5, pp. 3692–3704.
Bityurin, N., Znaidi, L., and Kanaev, A., Chem. Phys. Lett., 2003, vol. 374, nos. 1–2, pp. 95–99.
Kuznetsov, A.I., Kameneva, O., Alexandrov, A., et al., Phys. Rev. E, 2005, vol. 71, no. 2, pp. 021403-1–021403-7.
Bityurin, N., Kuznetsov, A.I., and Kanaev, A., Appl. Surf. Sci., 2005, vol. 248, nos. 1–4, pp. 86–90.
Savenije, T.J., Vermeulen, M.J.W., Haas, M.P., et al., Sol. Energy Mater. Sol. Cells., 2000, vol. 61, no. 1, pp. 9–18.
Kameneva, O.V., Kuznetsov, A.I., Smirnova, L.A., et al., Doklady Akad. Nauk, 2006, vol. 407, no. 1, pp. 29–31.
Salomatina, E.V., Bityurin, N.M., Gulenova, M.V., et al., J. Mater. Chem. C, 2013, vol. 1, pp. 6375–6385.
Kickelbick, G., Prog. Polym. Sci., 2003, vol. 28, pp. 83–114.
Schubert, U., Chem. Soc. Rev., 2011, vol. 40, pp. 587–582.
Mehrotra, R.C., Inorg. Chim. Acta. Rev., 1967, vol. 1, pp. 99–112.
Golubko, N.V., Yanovskaya, M.I., and Romm, I.P., et al., J. Sol-Gel Sci. Tech., 2001, vol. 20, pp. 245–262.
Pierre, A.S., Introduction to Sol-Gel Processing., Int. Ser. in Sol-Gel Processing: Technology and Applications, Dordrecht, 1998.
Kostin, A.S. and Kol’tsova, E.M., Fundamental’n. Issled., 2012, no. 9-2, pp. 381–387.
Mehrotra, R.C., J. Non-Cryst. Solids, 1990, vol. 121, nos. 1–3, pp. 1–6.
Barringer, E.A. and Bowen, H.K., Langmuir, 1985, vol. 1, no. 4, pp. 414–420.
Damm, C., J. Photochem. Photobiol. A: Chem., 2006, vol. 181, nos. 2–3, pp. 297–305.
Kabachii, Yu.A., Kochev, S.Yu., Blagodatskikh, I.V., et al., Polym. Sci., Ser. B., 2003, vol. 45, nos. 9–10, pp. 272–276.
Moskvichev, A.N. and Moskvichev, A.A., Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol., 2007, vol. 50, no. 3, pp. 69–71.
Afanas’ev, A.V., Moskvichev, A.N., Moskvichev, A.A., et al., Vestn. Nizhni Novgorod Gos. Univ., 2008, no. 3, pp. 60–64.
Moskvichev, A.N. and Moskvichev, A.A., Tr. Nizhegorod Gos. Tekh. Univ., Khim. Khim. Biotekhnol., 2007, vol. 80, no. 1, pp. 223–229.
Lushcheikin, G.A., Metody issledovaniya elektricheskikh svoistv polimerov (Methods for Study of Electrical Properties of Polymers), Moscow: Khimiya, 1988.
Chernov, I.A., Novikov, G.F., Dzhardimalieva, G.I., et al., Polym. Sci., Ser. A, 2007, vol. 49, no. 3, pp. 267–274.
Novitskii, S.P., Kenzin, V.I., and Voloshin, A.A., Elektrokhimiya, 1993, vol. 29, no. 1, pp. 138–143.
Barsucov, Ye. and Macdonald, R., Characterization of Materials, Kaufmann, E.N., Ed., John Wiley & Sons, 2012.
Damaskin, B.B. and Petrii, O.A., Vvedenie v elektrokhimicheskuyu kinetiku (Introduction to Electrochemical Kinetics), Moscow: Vysshaya Shkola, 1983.
Rodin, D.L., Solopchenko, A.V., Kepman, A.V., et al., Butlerov. soobshch., 2013, vol. 35, no. 8, pp. 31–41.
Rozenberg, B.A., Boiko, G.N., Bogdanova, L.M., et al., Polym. Sci. Ser. A, 2003, vol. 45, no. 9, pp. 819–825.
Arulin, V.I. and Efimov, L.I., Trudy Khim. Khim. Tekhnol., 1970, no. 2, pp. 74–77.
Metody analiza akrilatov i metakrilatov (Methods for Analysis of Acrylates and Methacrylates), Moscow: Khimiya, 1972.
Lipatov, Yu.S., Spravochnik po khimii polimerov (Handbook of Polymer Chemistry), Kiev: Naukova Dumka, 1971.
Solov’eva, L.M., Elektrodnye protsessy v galogenidnykh i okisnykh elektrolitakh (Electrode Processes in Halide and Oxide Electrolytes), Sverdlovsk: Ural. Nauchn. Tsentr Akad. Nauk SSSR, 1981, pp. 68–82.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Salomatina, A.N. Moskvichev, A.V. Knyazev, L.A. Smirnova, 2015, published in Zhurnal Prikladnoi Khimii, 2015, Vol. 88, No. 2, pp. 190–201.
Rights and permissions
About this article
Cite this article
Salomatina, E.V., Moskvichev, A.N., Knyazev, A.V. et al. Effect of kinetic features in synthesis of hybrid copolymers based on Ti(OPri)4 and hydroxyethyl methacrylate on their structure and properties. Russ J Appl Chem 88, 197–207 (2015). https://doi.org/10.1134/S1070427215020032
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427215020032