Abstract
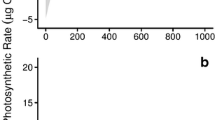
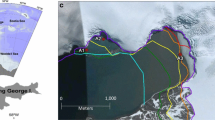
Complex investigations of the influence of environmental factors, viz., the temperature, photosynthetically active radiation (PAR), ambient seawater concentrations of ammonium (NH4), and orthophosphate (PO4), as well as the contents of organic carbon (C), nitrogen, phosphorus, and a-chlorophyll (Ch) on the rate of photosynthesis (Pn) and dark respiration (Rd) in the tissues of the unattached red seaweed Ahnfeltia tobuchiensis (Rhodophyta: Ahnfeltiales) population, were performed in the summers of 2000 and 2008 in Izmeny Bay (Kunashir Island) under in situ conditions. The dependence of photosynthesis on PAR intensity (P-I dependence) is described by the equation of a hyperbolic tangent. The population of A. tobuchiensis forms a layer up to 50 cm thick with an area of 23.3 km2 and a biomass of 125 000 tons. The Pn rate of seaweed population during daylight hours varies within a wide range, with an average of 1.04 mg O2 O2/(g dry weight h) and largely depends on PAR intensity and availability (r = 0.70–0.98). The maximum photosynthesis rate (Pmax) is substantially defined by the ambient concentration of NH4 (r 2 = 0.91, p < 0.01). The rate of Rd during the night is on average 0.1 mg O2/(g dry weight h) and mainly depends on the content of Ch in seaweed tissues (r 2 = 0.83, p < 0.01), which, in its turn, is regulated by the ambient concentration of PO4 (r 2 = 0.86, p < 0.01). With average biomass values of 5.4 kg/m2 or 1.8 kgdry weight/m2, the net primary production (Pn) of seaweed population is estimated to be on average 22.5 g O2/(m2 day) or 8.4 g C/(m2 day). Based on these indices, the investigated population is one of the most productive ecosystems of the World Ocean. It is supposed that such indices of the A. tobuchiensis population are attained due to the highly efficient use of weak light and a low light-saturation level of photosynthesis, compared to other seaweeds.
Similar content being viewed by others
References
Varfolomeeva, S.V., Titlyanov, E.A. and Cherbadzhi, I.I., Physiological Features of Competing Algae of the Ahnfeltia tobuchiensis Community, Biol. Morya, 1994, vol. 20, no. 1, pp. 34–41.
Vozzhinskaya, V.B., Bottom Vegetation: Biological Structure of the Ocean, Okeanologiya: Biologiya okeana (Oceanology: Biology of the Ocean), Moscow: Nauka, 1977, pp. 78–88.
Voskoboinikov, G.M., TEM Study of Ahnfeltia tobuchiensis Cells from Different Parts of the Thallus, Biologiya anfel’tsii (Biology of Ahnfeltia), Vladivostok: Dal’ Vost. Sci. Center, USSR Academy of Sciences, 1980, pp. 21–27.
Draper, N.R. and Smith, H. Applied Regression Analysis, New York: John Wiley & Sons, 1966.
Zvalinskii, V.I., Light and Temperature Conditions of the Inhabitation of Ahnfeltia in Starka Strait, Sea of Japan, Biologiya anfel’tsii (Biology of Ahnfeltia), Vladivostok: Dal’ Vost. Sci. Center, USSR Academy of Sciences, 1980, pp. 28–34.
Ivanova, M.B., Novozhilov, A.V. and Tsurpalo, A.P., Environmental Conditions and Some Peculiarities of Floral and Faunal Composition of Harvested Natural Fields of Ahnfeltia tobuchiensis in Starka Strait (Peter the Great Bay) and Izmeny Strait (Kunashir Island, the Kuril Islands), Biotekhnologicheskie osnovy akvakul’tury na Dal’nem Vostoke Rossii (Biotechnological Grounds of Aquaculture in Russian Far East), Vladivostok: Pacific Institute of Scientific Fisheries and Oceanography, 1994, pp. 83–99.
Kovardakov, S.A., Prazukin, A.V., Firsov, Yu.K., and Popov, A.E., Kompleksnaya adaptatsiya tsistoziry k gradientnym usloviyam (Complex Adaptations of Cystoseira to Gradient Conditions), Kiev: Naukova Dumka, 1985.
Kucheruk, N.V., Kononov, Yu.V., Luchina, N.P., and Maksimova, O.V., Effects of Biogenic Elements on Oxygen Exchange in Phyllophora nervosa and Phyllophora brodiaei and Its Ecological Significance, Biologiya chernomorskikh agarofitov (Biology of Black Sea Agarophytes), Moscow: Nauka, 1993. C. 63–69.
Makienko, V.F., On the History of Studies of Ahnfeltia plicata (Huds): Species of Ahnfeltia inhabiting along Far-Eastern Coasts of the USSR, Biologiya anfel’tsii (Biology of Ahnfeltia), Vladivostok: Dal’ Vost. Sci. Center, USSR Academy of Sciences, 1980, pp. 5–14.
Novozhilov, A.V., Vliyanie gidrodinamicheskikh uslovii na strukturu i produktivnost’ prirodnykh polei anfel’tsii tobuchinskoi (Effects of Hydrodynamic Conditions on Structure and Productivity in Natural Fields of Ahnfeltia tobuchiensis), Abstract of Cand. Sci. (Biol.) Dissertation, Vladivostok., 1989.
Odum, E.P., Ecology, 2nd Ed., New York: Holt, Rinehart and Winston, 1975.
Popova, L.I., Cherbadzhi, I.I. and Nekrasov, D.A., The Hydrochemical Regime of the Habitat of the Red Alga Ahnfeltia tobuchiensis Population in the Bay of Izmena (Kunashir Island), Russ. J. Mar. Biol., 2000, vol. 26, no. 5, pp. 350–356.
Propp, M.V. and Propp, L.N., Hydrochemical Indices and a-Chlorophyll Concentration in Coastal Waters of the Kuril Islands, Biol. Morya, 1988, no. 4, pp. 68–70.
Propp, L.N., Kashenko, S.D. and Propp, M.V., Determination of Major Biogenic Elements, Metody khimicheskogo analiza v gidrobiologicheskikh issledovaniyakh (Methods of Chemical Analysis in Hydrobiological Investigations), Vladivostok: Dal’ Vost. Nauch. Center, USSR Academy of Sciences, 1979, pp. 63–88.
Titlyanov, E.A., Le Nguyen Hieu, Nechai, E.G., Butorin, P.V., and Hoang Thi Linh, Daily Changes in Physiological Parameters of Photosynthesis and Dark Respiration in Algae of the Genus Sargassum from the Southern Regions of Vietnam, Biol. Morya, 1983, no. 3, pp. 39–48.
Titlyanov, E.A., Novozhilov, A.V. and Cherbadzhi, I.I., Anfel’tsiya tobuchinskaya: Biologiya, ekologiya, produktivnost’, (Ahnfeltia tobuchiensis: Biology, Ecology and Productivity), Moscow: Nauka, 1993.
Titlyanov, E.A., Cherbadzhi, I.I. and Chapman, D.J., Review on Biology, Productivity and Economical Potential of the Agariferous Red Alga Ahnfeltia tobuchiensis (Kanno et Matsubara) Mak. in the Seas of Russian Far East, Al’gologiya, 1999, vol. 9, no. 4, pp. 83–118.
Khailov, K.M. and Parchevskii, V.P., Ierarkhicheskaya regulyatsiya struktury i funktsii morskikh rastenii (Hierarchical Regulation of Structure and Function in Marine Plants), Kiev: Naukova dumka, 1983.
Cherbadzhi, I.I. and Popova, L.I., Effects of Environmental Factors on Oxygen Exchange in a Population of Ahnfeltia tobuchiensis (Kanno et Matsubara) Mak. (Ahnfeltiales, Rhodophyta), Al’gologiya, 2002, no. 2, pp. 222–233.
Cherbadzhi, I.I. and Propp, L.N., Photosynthesis and Respiration of a Deep-Water Periphyton Community (Macclesfield Bank, South China Sea), Biol. Morya, 2008, vol. 34, no. 5, pp. 351–358.
Cherbadzhi, I.I. and Titlyanov, E.A., Biology of Natural Monodominant Communities of the Red Alga Ahnfeltia tobuchiensis in the Far Eastern Seas of Russia, Russ. J. Mar. Biol., 1998, vol. 24, no. 2, pp. 67–76.
Cherbadzhi, I.I., Varfolomeeva, S.V. and Nekrasov, D.A., Seasonal Variations in the Production of Ahnfeltia tobuchiensis in the Stark Strait, Sea of Japan, Russ. J. Mar. Biol., 1995, vol. 21, no. 5, pp. 275–282.
Atkinson, M.J. and Smith, S.V., C:N:P Ratios of Benthic Marine Plants, Limnol. Oceanogr., 1983, vol. 28, pp. 568–574.
Cabello-Pasini and Alberte, R.S., Seasonal Patterns of Photosynthesis and Light-Independent Carbon Fixation in Marine Macrophytes, J. Phycol., 1997, vol. 33, pp. 321–329.
Cautinho, R. and Zingmark, R., Diurnal Photosynthetic Responses to Light by Macroalgae, J. Phycol., 1987, vol. 23, pp. 336–343.
Chapman, A.R.O., Nutrient Cycling in Marine Ecosystem, J. Limnol. Soc. Sth. Afr., 1986, vol. 12, pp. 22–42.
Chapman, A.R.O. and Craigie, J.S., Seasonal Growth in Laminaria longicruris: Relations with Dissolved Inorganic Nutrients and Internal Reserves of Nitrogen, Mar. Biol., 1977, vol. 40, pp. 197–205.
Cherbadgy, I.I. and Popova, L.I., Distribution, Biomass and Primary Production of Ahnfeltia tobuchiensis Population in the Bay of Izmena, Kunashir Island, Phycol. Res., 1998, vol. 46, pp. 1–10.
Conley, D.J., Biogeochemical Nutrient Cycles and Nutrient Management Strategies, Hydrobiologia, 1999, vol. 410, pp. 87–96.
Copertino, M.S., Cheshire, A. and Kildea, K., Photophysiology of a Turf Algal Community: Integrated Responses to Ambient Light and Standing Biomass, J. Phycol., 2009, vol. 45, pp. 324–336.
Fong, P., Donohoe, R.M. and Zedler, J.B., Nutrient Concentration in Tissue of the Macroalga Enteromorpha as a Function of Nutrient History: An Experimental Evaluation Using Field Microcosms, Mar. Ecol. Progr. Ser., 1994, vol. 106, pp. 273–281.
Haglund, K., Axelsson, L. and Pedersen, M., Photosynthesis and Respiration in the Alga Ahnfeltia plicata in a Flow-Through System, Mar. Biol., 1987, vol. 96, pp. 409–412.
Harrison, P.J. and Hurd, C.L., Nutrient Physiology of Seaweeds: Application of Concepts to Aquaculture, Cah. Biol. Mar., 2001, vol. 41, pp. 71–82.
Henley, W.J., Measurement and Interpretation of Photosynthetic Light-Response Curves in Algae in the Context of Photoinhibition and Diel Changes, J. Phycol., 1993, vol. 29, pp. 729–739.
Hernandez, I., Peralta, G., Perez-Llorens, J.L., et al., Biomass and Dynamics of Growth of Ulva Species in Pallmones River Estuary, J. Phycol., 1997, vol. 33, pp. 764–772.
Howarth, R.W., Nutrient Limitation of Net Primary Production in Marine Ecosystems, Ann. Rev. Ecol. Syst., 1988, vol. 19, pp. 89–110.
Hwang, R., Tsai, C. and Lee, T., Assessment of Temperature and Nutrient Limitation on Seasonal Dynamics among Species of Sargassum from a Coral Reef in Southern Taiwan, J. Phycol., 2004, vol. 40, pp. 463–473.
Jassby, A.D. and Platt, T., Mathematical Formulation of the Relationship between Photosynthesis and Light for Phytoplankton, Limnol. Oceanogr., 1976, vol. 21, pp. 540–547.
Jeffrey, S.W. and Humphrey, G.F., New Spectrophotometric Equations for Determining Chlorophylls a, b, c 1 and c 2 in Higher Plants, Algae and Natural Phytoplankton, Biochem. Physiol. Pflanz., 1975, vol. 167, pp. 191–194.
Johansson, G. and Snoeijs, P., Macroalgal Photosynthetic Responses to Light in Relation to Thallus Morphology and Depth Zonation, Mar. Ecol. Progr. Ser., 2002, vol. 244, pp. 63–72.
Komfeldt, R.-A., Relation Between Nitrogen and Phosphorus Content of Macroalgae and the Water of Northern Oresund, Bot. Mar., 1982, vol. 25, pp. 197–201.
Lapointe, B.E. and Tenore, K.R., Experimental Outdoor Studies with Ulva fasciata Delile. I. Interaction of Light and Nitrogen on Nutrient Uptake, Growth, and Biochemical Composition, J. Exp. Mar. Biol. Ecol., 1981, vol. 53, pp. 135–152.
Lapointe, B.E., Barile, P.J., Yentsch, C.S., et al., The Relative Importance of Nutrient Enrichment and Herbivory on Macroalgal Communities near Norman’s Pond Cay, Exumas Cays, Bahamas: A “Natural” Enrichment Experiment, J. Exp. Mar. Biol. Ecol., 2004, vol. 298, pp. 275–301.
Lapointe, B.E., Barile, P.J., Littler, M.M., et al., Macroalgal Blooms on Southeast Florida Coral Reefs I. Nutrient Stoichiometry of the Invasive Green Alga Codium isthmocladum in the Wider Caribbean Indicates Nutrient Enrichment, Harmf. Alg., 2005, vol. 4, pp. 1092–1105.
Lapointe, B.E., Littler, M.M. and Littler, D.S., Nutrient Availability to Marine Macroalgae in Siliciclastic Versus Carbonate-Rich Coastal Waters, Estuaries, 1992, vol. 15, no. 1, pp. 75–82.
Littler, M.M., Littler, D.S., Blair, S.M. and Norris, J.N., Deep-Water Plant Communities from an Unchartered Seamount off San Salvador Island, Bahamas: Distribution, Abundance, and Primary Productivity, Deep-Sea Res., 1986, vol. 33, pp. 881–892.
Littler, M.M., Littler, D.S. and Hanisak, M.D., Deep-Water Rhodolith Distribution, Productivity and Growth History at Sites of Formation and Subsequent Degradation, J. Exp. Mar. Biol. Ecol., 1991, vol. 150, pp. 163–182.
Lüning, K., Seaweeds: Their Environment, Biogeography, and Ecophysiology, New York: Wiley-Liss, 1990.
Maxwell, A.E., Multivariate Analysis in Behavioural Research, London: Chapman and Hall, 1977.
Menendez, M., Herrera, J. and Comin, F.A., Effect of Nitrogen and Phosphorus Supply on Growth, Chlorophyll Content and Tissue Composition of the Macroalga Chaetomorpha linum (O.F. Mull.) Kutz in a Mediterranean Coastal Lagoon, Sci. Mar., 2002, vol. 66, pp. 355–364.
Parker, H.S., Effect of Simulated Current on the Growth Rate and Nitrogen Metabolism of Gracilaria tikvahiae (Rhodophyta), Sci. Mar., 1982, vol. 69, no. 2, pp. 137–145.
Pedersen, M.F. and Borum, J., Nutrient Control of Algal Growth in Estuarine Waters. Nutrient Limitation and the Importance of Nitrogen Requirements and Nitrogen Storage among Phytoplankton and Species of Macroalgae, Mar. Ecol. Progr. Ser., 1996, vol. 142, pp. 261–272.
Phillips, J.C. and Hurd, C.L., Kinetics of Nitrate, Ammonium, and Urea Uptake by Four Intertidal Seaweeds from New Zealand, J. Phycol., 2004, vol. 40, pp. 534–545.
Propp, M.V., Garber, M.R. and Ryabushko, V.J., Unstable Processes in the Metabolic Rate Measurement in Flow-Through System, Mar. Biol., 1982, vol. 67, pp. 47–51.
Ramus, J., Productivity of Seaweeds, Primary Productivity and Biogeochemical Cycles in the Sea, New York: Plenum Press, 1992, pp. 239–255.
Raven, J.A., Beardall, J., Johnston, A.M., et al., Inorganic Carbon Acquisition by Hormosira banksii (Phaeophyta: Fucales) and Its Epiphyte Notheia anomala (Phaeophyta: Fucales), Phycologia, 1995, vol. 34, pp. 267–277.
Redfield, A.C., Ketchum, B.H. and Richards, F.A., The Influence of Organisms on the Composition of Sea-Water, The Sea, New York: Wiley Interscience, 1963, vol. 2, pp. 26–77.
Rosenberg, G. and Ramus, J., Ecological Growth Strategies in the Seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): Soluble Nitrogen and Reserve Carbohydrates, Mar. Biol., 1982, vol. 66, pp. 251–259.
Smit, A.J., Nitrogen Uptake by Gracilaria gracilis (Rhodophyta): Adaptations to a Temporally Variable Nitrogen Environment, Bot. Mar., 2002, vol. 45, no. 2, pp. 196–209.
Strickland, J.D.H. and Parsons, T.R., A Practical Handbook of Seawater Analysis, Bull. Fish. Res. Board Can., 1972, vol. 167, pp. 1–311.
Thomas, T.E. and Harrison, P.J., Rapid Ammonium Uptake and Nitrogen Interactions in Five Intertidal Seaweeds Grown under Field Conditions, J. Exp. Mar. Biol. Ecol., 1987, vol. 107, no. 1, pp. 1–8.
Titlyanov, E. A. and Cherbadgy, I.I., Biology, Productivity and Extensive Culture of the Agarophyte, Ahnfeltia tobuchiensis (Ahnfeltiales, Rhodophyta), in the Seas of the Far East, The Yellow Sea, 1999, vol. 5, pp. 59–72.
Wheeler, P.A. and Bjornsater, B.R., Seasonal Fluctuations in Tissue Nitrogen, Phosphorus, and N:P for Five Macroalgal Species Common to the Pacific Northwest Coast, J. Phycol., 1992, vol. 28, pp. 1–6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.I. Cherbadgy, L.I. Sabitova, V.A. Parensky, 2010, published in Biologiya Morya.
The article was translated by the authors.
Rights and permissions
About this article
Cite this article
Cherbadgy, I.I., Sabitova, L.I. & Parensky, V.A. The influence of environmental factors and nutrient concentrations in tissues of the seaweed Ahnfeltia tobuchiensis (Rhodophyta: Ahnfeltiales) on the primary production and dark respiration of its population. Russ J Mar Biol 36, 282–292 (2010). https://doi.org/10.1134/S1063074010040061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074010040061