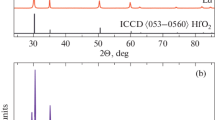
Abstract
In this work, a high-density ceramics Ln2Hf2O7 (Ln = La, Nd, Sm, Eu, Gd) were synthesized by mechanical activation followed by high-temperature synthesis at 1600°C (3–10 h) and their transport properties were compared with those of Ln2.1Hf1.9O6.95 (Ln = La, Nd, Sm, Eu) doped solid solutions. The total conductivity of ceramics was studied using impedance spectroscopy and dc four-probe method; for Ln2Hf2O7 (Ln = Sm, Eu), by determining the total conductivity as a function of oxygen partial pressure. The maximum oxygen-ion conductivity was observed for Gd2Hf2O7 (~1 × 10–3 S/cm at 700°C); it was shown to approach the conductivity of Gd2Zr2O7 (~2 × 10–3 S/cm at 700°C) for the first time. Thus, the gadolinium hafnate can be a promising material for further doping in order to obtain highly conductive electrolytes. Among pure rare-earth hafnates, the proton conductivity was reliably observed for Nd2Hf2O7 only; however, ac measurements detected low-temperature proton conductivity in the Gd2Hf2O7 up to 450°С as well. With a decrease in the lanthanide ionic radius, the oxygen-ion conductivity increased in the Ln2Hf2O7 (Ln = La, Nd, Sm, Gd) series. Although the conductivity of samarium hafnate is an order of magnitude lower than that of Gd2Hf2O7, it has a wide range of oxygen-ion conductivity (~10–18–1 atm at 700, 800°C); there is no contribution from hole conductivity in air, in contrast to Eu2Hf2O7. Among doped Ln2.1Hf1.9O6.95 pyrochlore solid solutions (Ln = La, Nd, Sm, Eu), the proton conductivity of ~8 × 10−5 S/cm at 700°C was shown in Ln2.1Hf1.9O6.95 (Ln = La, Nd). With a decrease in the lanthanide ionic radius, the proton conductivity disappeared; the oxygen-ion one, increased.






Similar content being viewed by others
REFERENCES
Diaz-Guillen, J.A., Fuentes, A.F., Díaz-Guillen, M.R., Almanza, J.M., Santamarıa, J., and Leon, C., The effect of homovalent A-site substitutions on the ionic conductivity of pyrochlore-type Gd2Zr2O7, J. Power Sources, 2009, vol. 186, no. 2, p. 349.
Yamamura, H., Nishino, H., Kakinuma, K., and Nomura, K., Electrical conductivity anomaly around fluorite-pyrochlore phase boundary, Solid State Ionics, 2003, vol. 158, p. 359.
Vassen, R., Cao, X., Tietz, F., Basu, D., and Stover, D., Zirconates as new materials for thermal barrier coatings, J. Amer. Ceram. Soc., 2000, vol. 83, p. 2023.
Lopez-Cota, F.A., Cepeda-Sanchez, N.M., Díaz-Guillen, J.A., Dura, O.J., Lopez de la Torre, M.A., Maczka, M., Ptak, M., and Fuentes, A.F., Electrical and thermophysical properties of mechanochemically obtained lanthanide hafnates, J. Amer. Ceram. Soc., 2017, p. 1. https://doi.org/10.1111/jace.14712
Shlyakhtina, A.V. and Shcherbakova, L.G., New solid electrolytes of the pyrochlore family, Russ. J. Electrochem., 2012, vol. 48, p. 1. https://doi.org/10.1134/S1023193512010144
Mullens, B.G., Zhang, Z., Avdeev, M., Brand, H.E.A., Cowie, B.C.C., Muzquiz, M.S., and Kennedy, B.J., Effect of long and short-range disorder on the oxygen ionic conductivity of Tm2(Tm2 – xTmx)O7 – x/2 “stuffed” pyrochlores, Inorg. Chem., 2021, vol. 60, p. 4517. https://doi.org/10.1021/acs.inorgchem.0c03363
Mullens, B.G., Zhang, Z., Avdeev, M., Brand, H.E.A., Cowie, B.C.C., Muzquiz, M.S., and Kennedy, B.J., Average and local ordering of Yb2(Ti2 – xYbx)O7 – x/2 ‘stuffed’ pyrochlores: The development of a robust structural model, J. Solid State Chem., 2021, vol. 302, p. 122412. https://doi.org/10.1016/j.jssc.2021.122412
Lyskov, N.V., Shchegolikhin, A.N., Stolbov, D.N., Kolbanev, I.V., Gomes, E., Abrantes, J.C.C., and Shlyakhtina, A.V., Study of oxygen-ion conductivity and luminescence in the ZrO2–Nd2O3 system: impact of local heterogeneity, Electrochim. Acta, 2022, vol. 403, p. 139632.
Shlyakhtina, A.V., Belov, D.A., Karyagina, O.K., and Shcherbakova, L.G., Ordering processes in Ln2TiO5 (Ln = Dy – Lu): The role of thermal history, J. Alloys Compd., 2009, vol. 479, p. 6.
Shlyakhtina, A.V., Belov, D.A., Stefanovich, S.Y., and Shcherbakova, L.G., Nanostructuring phenomena in oxygen-conducting complex oxides of heavy REE, Russ. J. Electrochem., 2011, vol. 47, p. 620.
Zvonareva, I., Fu, X.-Z., Medvedev, D., and Shao, Z., Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes, Energy Environ. Sci., 2022, vol. 15, p. 439. https://doi.org/10.1039/D1EE03109K
Medvedev, D.A., Current drawbacks of proton-conducting ceramic materials: How to overcome them for real electrochemical purposes, Curr. Opin. Green Sustain. Chem., 2021, vol. 32, p. 100549. https://doi.org/10.1016/j.cogsc.2021.100549
Kasyanova, A.V., Radenko, A.O., Lyagaeva, Y.G., and Medvedev, D.A., Lanthanum-Containing Proton-Conducting Electrolytes with Perovskite Structures, Membr. and Membr. Technol., 2021, vol. 3, p. 73. https://doi.org/10.1134/S2517751621020050
Hagiwara, T., Yamamura, H., and Nishino, H., Relationship between oxide-ion conductivity and ordering of oxygen vacancy in the Ln2Zr2O7 (Ln = La, Nd and Eu) system using high temperature XRD, J. Fuel Cell Sci. Technol., 2011, vol. 8, p. 051020.
Xia, X.L., Gao, S., Liu, Z.G., and Ouyang, J.H., The influence of pentavalent Nb substitution for Zr on electrical property of oxide-ion conductor Gd2Zr2O7, Electrochim. Acta., 2010, vol. 55, no. 19, p. 5301.
Anokhina, I.A., Animitsa, I.E., Voronin, V.I., Vykhodets, V.B., Kurennykh, T.E., Molchanova, N.G., Vylkov, A.I., Dedyukhin, A.E., and Zaikov, Y.P., The structure and electrical properties of lithium doped pyrochlore Gd2Zr2O7, Ceram. Intern., 2021, vol. 47, p. 1949.
Sharma, S.K., Mohanty, H.S., Pradhan, D.K., Kumar, A., Shukla, V.K., Singh, F., and Kulriya, P.K., Structural, dielectric and electrical properties of pyrochlore-type Gd2Zr2O7 ceramic, J. Mater. Sci: Mater. Electron., 2020, vol. 31, p. 21959.
Shlyakhtina, A.V., Gorshkov, N.V., Kolbanev, I.V, Shefer, K.I., Kasyanova, A.V., and Medvedev, D.A., Electrical properties of Gd2Zr2O7 doped with beryllium, Neorgan. materialy (in Russian), 2021, vol. 57, no. 11, p. 1253. https://doi.org/10.31857/S0002337X21110117
Liu, Z.G., Gao, S., Ouyang, J.H., and Xia, X.L., Influence of MoO3 doping on structure and electrical conductivity of defect fluorite-type Gd2Zr2O7, J. Alloys Compd., 2010, vol. 506, p. 868. https://doi.org/10.1016/j.jallcom.2010.07.101
Anithakumari, P., Grover, V., Nandi, C., Bhattacharyya, K., and Tyagi, A.K., Utilizing non-stoichiometry in Nd2Zr2O7 pyrochlore: exploring superior ionic conductors, RSC Adv., 2016, vol. 6, p. 97566. https://doi.org/10.1039/C6RA08722A
Shimura, T., Komori, M., and Iwahara, H., Ionic conduction in pyrochlore-type oxides containing rare earth elements at high temperature, Solid State Ionics, 1996, vol. 86, p. 685.
Omata, T. and Otsuka-Yao-Matsuo, S., Electrical properties of proton-conducting Ca2+-doped La2Zr2O7 with a pyrochlore-type structure, J. Electrochem. Soc., 2001, vol. 148, p. 252.
Omata, T., Ikeda, K., Tokashiki, R., and Otsuka-Yao-Matsuo, S., Proton solubility for La2Zr2O7 with a pyrochlore structure doped with a series of alkaline-earth ions, Solid State Ionics, 2004, vol. 167, p. 389.
Labrincha, J.A., Frade, J.R., and Marques, F.M.B., Protonic conduction in La2Zr2O7-based pyrochlore materials, Solid State Ionics, 1997, vol. 99, p. 33.
Antonova, E.P., Farlenkov, A.S., Tropin, E.S., Eremin, V.A., Khodimchuk, A.V., and Ananiev, M.V., Oxygen isotope exchange, water uptake and electrical conductivity of Ca-doped lanthanum zirconate, Solid State Ionics, 2017, vol. 306, p. 112.
Shlyakhtina, A.V., Abrantes, J.C.C., Gomes, E., Lyskov, N.V., Konysheva, E.Yu., Chernyak, S.A., Kharitonova, E.P., Karyagina, O.K., Kolbanev, I.V., and Shcherbakova, L.G., Evolution of oxygen-ion and proton conductivity in Ca doped Ln 2Zr2O7 (Ln = Sm, Gd), located near pyrochlore – fluorite phase boundary, Materials, 2019, vol. 12, p. 2452.
Eurenius, K.E.J., Ahlberg, E., and Knee, C.S., Role of B-site ion on proton conduction in acceptor-doped Sm2B2O7 – δ (B = Ti, Sn, Zr and Ce) pyrochlores and C-type compounds, Dalton Trans., 2011, vol. 40, p. 3946. https://doi.org/10.1039/c0dt01347a
Shlyakhtina, A.V., Lyskov, N.V., Konysheva, E.Yu., Chernyak, S.A., Kolbanev, I.V., Vorobieva, G.A., and Shcherbakova, L.G., Gas-tight proton-conducting Nd2 – xCaxZr2O7 – δ (x = 0, 0.05) ceramics, J. Solid State Electrochem., 2020, vol. 24, p. 1475. DOI.org/10.1007/s10008-020-04574-6
Eurenius, K.E.J., Ahlberg, E., Ahmed, I., Eriksson, S.G., and Knee, C.S., Investigation of proton conductivity in Sm1.92Ca0.08Ti2O7 – δ and Sm2Ti1.92Y0.08O7 – δ pyrochlores, Solid State Ionics, 2010, vol. 181, p. 148.
Kiruthika, G.V.M., Govindan Kutty, K.V., and Varadarju, U.V., Effect of aliovalent ion substitution on the oxide ion conductivity in rare-earth pyrohafnates RE2 – xSrxHf2O7 – δ and RE2Hf2 – xAlxO7 – δ (RE = Gd and Nd; x = 0, 0.1, and 0.2), Solid State Ionics, 1998, vol. 110, p. 335. https://doi.org/10.1016/S0167-2738(98)00140-4
Cepeda-Sanchez, N.M., Dias-Guillen, J.A., Macka, M., Amador, U., and Fuentes, A., Mechanochemical synthesis, crystal structure and ion conduction in the Gd2Hf2 – xTixO7 system, J. Mater. Sci., 2017, vol. 52, p. 11933. https://doi.org/10.1007/s10853-017-1037-2
Cepeda-Sanchez, N.M., Fuentes, A.F., Lopez-Cota, F.A., Rodrigues-Reyes, M., and Dias-Guillen, J.A., Mechanochemical synthesis and electrical properties of Gd2Hf2 – xZrxO7 solid electrolytes for their use in SOFC’s, J. Appl. Electrochem., 2015, vol. 45, p. 1231. https://doi.org/10.1007/s10800-015-0828-x
Sardar, S., Kale, G., and Ghadiri, M., Microstructure and impedance spectroscopy of high density holmium hafnate (Ho2Hf2O7) from nanoparticulate compacts, Mater. Sci. Eng. B, 2021, vol. 265, p. 114989. https://doi.org/10.1016/j.mseb.2020.114989
Shlyakhtina, A.V., Lyskov, N.V., Shchegolikhin, A.N., Kolbanev, I.V., Chernyak, S.A., and Konysheva, E.Yu., Valence state of europium and samarium in Ln2Hf2O7 (Ln = Eu, Sm) based in oxygen—ion conductors, Ceram. Intern., 2021, vol. 47, p. 26898. https://doi.org/10.1016/j.ceramint.2021.06.099
Rejith, R.S., Sam Solomon, Influence of pyrochlore domains on the structure and electrical properties Gd2 – xDyxZr1.5Hf0.5O7 energy materials, J. Alloys Compounds, 2021, vol. 855, p. 157291.
Mikuśkiewicz, M., Migas, D., and Moskal, G., Synthesis and thermal properties of zirconate, hafnate and cerate of samarium, Surface Coatings Technol., 2018, vol. 354, p. 66. https://doi.org/10.1016/j.surfcoat.2018.08.096
Matovic, B., Maletaskic, J., Bucevac, D., Zagorac, J., Fajar, M., Yoshida, K., and Yano, T., Synthesis, characterization and sintering of Gd2Hf2O7 powders synthesized by solid state displacement reaction at low temperature, Ceram. Intern., 2018, vol. 44, p. 16972. https://doi.org/10.1016/j.ceramint.2018.06.138
Mikuśkiewicz, M., Moskal, G., Migas, D., and Stopyr, M., Thermal diffusivity characterization of europium zirconate, cerate and hafnate, Ceram. Int., 2019, vol. 45, p. 2760. https://doi.org/10.1016/j.ceramint.2018.07.301
Shlyakhtina, A.V., Lyskov, N.V., Shchegolikhin, A.N., Chernyak, S.A., Knotko, A.V., Kolbanev, I.V., and Shcherbakova, L.G., Structure evolution, ionic and proton conductivity of solid solutions based on Nd2Hf2O7, Ceram. Intern., 2020, vol. 46, p. 17383. https://doi.org/10.1016/j.ceramint.2020.04.029
Shlyakhtina, A.V., Lyskov, N.V., Nikiforova, G.E., Kasyanova, A.V., Vorobieva, G.A., Kolbanev, I.V., Stolbov, D.N., and Medvedev, D.A., Proton conductivity of La2(Hf2 – xLax)O7 – x/2 “stuffed” pyrochlores, Appl. Sci., 2022, vol. 12, p. 4342. https://doi.org/10.3390/app12094342
ZView (Scribner Associates Inc., USA).
Shlyakhtina, A.V. and Pigalskiy, K.S., Tolerance factor as the basic criterion in searching for promising oxygen-ion and proton conductors among Ln2 – xDxM2O7 – δ (Ln = La – Lu; M = Sn, Ti, Zr, Hf; D = Sr, Ca, Mg; x = 0, 0.1) 3+/4+ pyrochlores, Mater. Res. Bull., 2019, vol. 116, p. 72. https://doi.org/10.1016/j.materresbull.2019.04.021
Kreller, C.R. and Uberuaga, B.P., The role of cation ordering and disordering on mass transport in complex oxides, Current Opinion Solid State Mater. Sci., 2021, vol. 25, p. 100899. https://doi.org/10.1016/j.cossms.2021.100899
Subramanian, M.A., Aravamudan, G., and Subba Rao, G.V., Oxide pyrochlores – a review, Progress. Solid State Chem., 1983, vol. 15, p. 55. https://doi.org/10.1016/0079-6786(83)90001-8
Funding
This study was subsidized by the Ministry of Sciences and Higher Education of the Russian Federation in frames of the Semenov Federal Research Center for Chemical Physics State contract “Next-generation nanostructured systems with unique functional properties” (reg. no. 122040500071-0). The material conductivity study is performed in part in frames of the Federal Research Center of Problems of Chemical Physics and Medicinal Chemistry contract (the State reg. no. АААА-А19-119061890019-5).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by Yu. Pleskov
Based on the materials of the 16th International Meeting “Fundamental Problems of Solid State Ionics,” Chernogolovka, June 27–July 7, 2022.
Rights and permissions
About this article
Cite this article
Shlyakhtina, A.V., Lyskov, N.V., Kolbanev, I.V. et al. Proton and Oxygen-Ion Conductivity of the Pure and Lanthanide-Doped Hafnates with Pyrochlore Structure. Russ J Electrochem 59, 449–460 (2023). https://doi.org/10.1134/S1023193523060058
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193523060058