Abstract
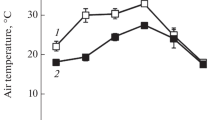
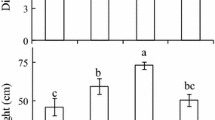
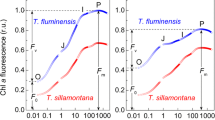
Morphological and functional characteristics of Plantago media L. leaves were compared for plants growing at different light regimes on limestone outcrops in Southern Timan (62°45′N, 55°49′E). The plants grown in open areas under exposure to full sunlight had small leaves with low pigment content and high specific leaf weight; these leaves exhibited high photosynthetic capacity and elevated water use efficiency at high irradiance. The maximum photochemical activity of photosystem II (F v/F m) in leaves of sun plants remained at the level of about 0.8 throughout the day. The photosynthetic apparatus of sun plants was resistant to excess photosynthetically active radiation, mostly due to non-photochemical quenching of chlorophyll fluorescence (qN). This quenching was promoted by elevated deepoxiation of violaxanthin cycle pigments. Accumulation of zeaxanthin, a photoprotective pigment in sun plant leaves was observed already in the morning hours. The plant leaves grown in the shade of dense herbage were significantly larger than the sun leaves, with pigment content 1.5–2.0 times greater than in sun leaves; these leaves had low qN values and did not need extensive deepoxidation of violaxanthin cycle pigments. The data reveal the morphophysiological plasticity of plantain plants in relation to lighting regime. Environmental conditions can facilitate the formation of the ecotype with photosynthetic apparatus resistant to photoinhibition. Owing to this adjustment, hoary plantain plants are capable of surviving in ecotopes with high insolation.
Similar content being viewed by others
Abbreviations
- Ant:
-
antheraxanthin
- β-Car:
-
β-carotene
- Car:
-
carotenoids
- Chl:
-
chlorophyll
- Ft, F0, and Fm:
-
stationary minimum, and maximum yields of chlorophyll fluorescence, respectively
- Fv/Fm:
-
variable to maximum fluorescence ratio, i.e., the maximum quantum yield of PSII
- LCFA:
-
long-chain fatty acids
- MDA:
-
malondialdehyde
- PAR:
-
photosynthetically active radiation
- P n :
-
rate of apparent (net) photosynthesis
- PSII:
-
photosystem II
- PSA:
-
photosynthetic apparatus
- qP and qN:
-
coefficients of photochemical and non-photochemical fluorescence quenching, respectively
- Vio:
-
violaxanthin
- VXC:
-
violaxanthin cycle
- Y:
-
effective quantum yield of PSII photochemistry, i.e., the part of light energy used by PSII for electron transport
- Zea:
-
zeaxanthin
References
Lyubimenko, V.N., Photosynthesis and Plant Adaptation to Light, Selected Works, vol. 1, Lyubimenko, V.N., Ed., Kiev: Akad. Nauk UkrSSR, 1963.
Bazzaz, F.A. and Carlson, R.W., Photosynthetic Acclimation to Variability in Light Environment of Early and Late Successional Plant, Oecologia, 1982, vol. 54, pp. 3313–3316.
Osmond, B. and Forster, B., Photoinhibition: Then and Now, Photoprotection, Photoinhibition, Gene Regulation, and Environment, Demmig-Adams, B., Adams, W.W., III., and Mattoo, A.K., Eds., Dordrecht: Springer-Verlag, 2006, pp. 11–22.
Murata, N., Takahashi, S., Nishiyama, Y., and Allakhverdiev, S.I., Photoinhibition of Photosystem II under Environmental Stress, Biochim. Biophys. Acta, 2007, vol. 1767, pp. 414–421.
Kuiper, D. and Smid, A., Genetic Differentiation and Phenotypic Plasticity in Plantago major ssp. major: 1. The Effect of Differences in Level of Irradiance on Growth, Photosynthesis, Respiration and Chlorophyll Content, Physiol. Plant., 1985, vol. 60, pp. 520–528.
Lambers, H., Posthumus, F., Stulen, I., Lanting, I., van de Dijk, S.J., and Hofstra, R., Energy Metabolism of Plantago major ssp. major as Dependent on the Supply of Mineral Nutrients, Physiol. Plant., 1981, vol. 51, pp. 245–252.
Mudrik, V., Kosobrukhov, A., Knyazeva, I., and Pigulevskaya, T., Changes in the Photosynthetic Characteristics of Plantago major Plants Caused by Soil Drought Stress, Plant Growth Regul., 2003, vol. 40, pp. 1–6.
Garmash, E.V. and Golovko, T.K., CO2 Gas Exchange and Growth in Rhaponticum carthamoides under the Conditions of the Middle Taiga Subzone of Northeastern Europe: 1. Dependence of Photosynthesis and Respiration on Environmental Factors, Russ. J. Plant Physiol., 1997, vol. 44, pp. 737–745.
Krause, G.H. and Weis, E., Chlorophyll Fluorescence and Photosynthesis: The Basis, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1991, vol. 42, pp. 313–349.
Lichtenthaler, H., Buschmann, C., and Knapp, M., Measurement of Chlorophyll Fluorescence Kinetics (Kautsky Effect) and the Chlorophyll Fluorescence Decrease Ratio (RFD-Values) with the PAM-Fluorometer, Analytical Methods in Plant Stress Biology, Filek, M., Biesaga-Kos-cielniak, J., and Marcin-ska, I., Eds., Krako-w: Inst. Plant Physiol., Polish Acad. Sci., 2004, pp. 93–111.
Maslova, T.G., Popova, I.A., and Popova, O.F., Spectrophotometry for Carotenoid Quantification: Critical Evaluation, Sov. Plant Physiol., 1986, vol. 33, pp. 615–619.
Kornyushenko, G.A. and Sapozhnikov, D.I., Determination of Carotenoids in Green Leaf Using Thin-Layer Chromatography, Metody kompleksnogo izucheniya fotosinteza (Methods for Complex Investigation of Photosynthesis), Leningrad: Vses. Inst. Rastenievod., 1969, pp. 181–192.
Schindler, C. and Lichtenthaler, H.K., Photosynthetic CO2-Assimilation, Chlorophyll Fluorescence and Zeaxanthin Accumulation in Field Grown Maple Trees in the Course of a Sunny and Cloudy Day, J. Plant Physiol., 1996, vol. 148, pp. 399–412.
Glyad, V.M., Determination of Monosaccharides, Disaccharides, and Oligosaccharides in the Same Plant Sample by High-Performance Liquid Chromatography, Russ. J. Plant Physiol., 2002, vol. 49, pp. 277–282.
Lukatkin, A.S. and Golovanova, V.S., Lipid Peroxidation Intensity in Chilled Leaves of Thermophyte Plants, Sov. Plant Physiol., 1988, vol. 35, pp. 773–780.
Aro, E.-M., Virgin, I., and Andersson, B., Photoinhibition of Photosystem II: Inactivation, Protein Damage, and Turnover, Biochim. Biophys. Acta, 1993, vol. 1143, pp. 113–134.
Krasnovsky, A.A., Singlet Oxygen: Mechanisms of Development and Pathways of Deactivation in Biological Systems, Biofizika, 1994, vol. 39, pp. 236–250.
Polesskaya, O.G., Rastitel’naya kletka i aktivnye formy kisloroda (Plant Cell and Reactive Oxygen Species), Moscow: Knizhn. Dom Univ., 2007.
Adams, W.W., III, Zarter, C.R., Much, K.E., Amiard, V., and Demmig-Adams, B., Energy Dissipation and Photoinhibition: A Continuum of Photoprotection, Photoprotection, Photoinhibition, Gene Regulation, and Environment, Demmig-Adams, B., Adams, W.W., III., and Mattoo, A.K., Eds., Dordrecht: Springer-Verlag, 2006, pp. 49–64.
Demmig-Adams, B., Linking the Xanthophyll Cycle with Thermal Energy Dissipation, Photosynth. Res., 2003, vol. 76, pp. 73–80.
Genty, B., Briantais, J.M., and Baker, N.R., The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence, Biochim. Biophys. Acta, 1989, vol. 990, pp. 87–92.
Rmiki, N.-E., Lemoine, Y., and Schoefs, B., Carotenoids and Stress in Higher Plants and Algae, Handbook of Plant and Crop Stress, Pessarakli, M., Ed., New York: Marcel Dekker, 1999, pp. 465–482.
Gruszeski, W.I., Carotenoids in Membranes, Advances in Photosynthesis, vol. 8, The Photochemistry of Carotenoids, Frank, H.A., Young, A.J., Britton, G., and Cogdell, R.J., Eds., The Netherlands: Kluwer, 1999, pp. 363–379.
Dymova, O.A. and Golovko, T.K., Light Adaptation of Photosynthetic Apparatus in Ajuga reptans L., a Shade-Tolerant Plants as an Example, Russ. J. Plant Physiol., 2007, vol. 54, pp. 440–446.
Adams, W.W., Demmig-Adams, B., Rosenstiel, T.N., Brightwell, A.K., and Ebbert, V., Photosynthesis and Photoprotection in Overwintering Plants, Plant Biol., 2002, vol. 4, pp. 545–557.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.K. Golovko, I.V. Dalke, I.G. Zakhozhiy, O.V. Dymova, G.N. Tabalenkova, 2011, published in Fiziologiya Rastenii, 2011, Vol. 58, No. 4, pp. 490–501.
Rights and permissions
About this article
Cite this article
Golovko, T.K., Dalke, I.V., Zakhozhiy, I.G. et al. Functional plasticity of photosynthetic apparatus and its resistance to photoinhibition in Plantago media . Russ J Plant Physiol 58, 549–559 (2011). https://doi.org/10.1134/S1021443711040054
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443711040054