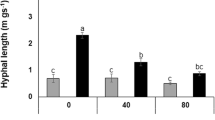
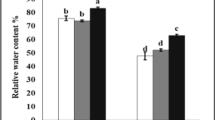
Abstract
Salt stress is considered as one of the most important abiotic factors limiting plant growth and yield in many areas of the world. It has been shown that Vesicular Arbuscular Mycorrhizal Fungi (AMF) can alleviate this deficiency. The effects of AMF inoculation on growth variables and mineral nutrition of Carthamus tinctorius L. under salt stress condition were studied. Plants were grown in a sterilized, low-P sandy soil with Glomus etunicatum inoculum (10–12 spore/g soil) in a greenhouse. RLC (Root Length Colonized) percent was higher in control plants than treated ones with different salt concentrations. Shoot and root weights, height, the number of leaves, the number of lateral branches, and also leaf area of mycorrhizal (M) plants were higher than nonmycorrhizal (NM) ones in both controlled and salt-treated plants. P, Zn, Fe, Ca, K, Cu, and N contents in M plants were higher than in NM plants in control, low and medium salinity conditions, but Na content was lower in aerial parts of the M plants. The results showed a higher tolerance of inoculated M plants toward salt stress and their better growth.
Similar content being viewed by others
Abbreviations
- AMF:
-
arbuscular micorrhizal fungi
- RLC:
-
root length colonized
- M:
-
mycorrhizal plants
- NM:
-
nonmycorrhizal plants
References
Niu, X., Bressan, R.A., Hasegawa, PP.M., and Pardo, J.M., Ion Homeostasis in NaCl Stress Environments, Plant Physiol., 1995, vol. 109, pp. 735–742.
Al-Karaki, G.N., Growth, Water Use Efficiency, and Mineral Acquisition by Tomato Cultivars Grown under Salt Stress, J. Plant Nutr., 2000, vol. 23, pp. 1–8.
Heijden, M.G.A., Klirronomos, J.N., Ursic, M., and Sanders, I.R., Mycorrhizal Fungal Diversity Determines Plant Biodiversity, Ecosystem Variability and Productivity, Nature, 1998, vol. 396, pp. 69–72.
George, E., Marschner, H., and Jakobsen, I., Role of Arbuscular Mycorrhizal Fungi in Uptake of Phosphorus and Nitrogen from Soil, Crit. Rev. Biotechnol., 1995, vol. 15, pp. 257–270.
Smith, S.E. and Gianinazzi-Pearson, V., Physiological Interactions between Symbionts in AM Plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1988, vol. 39, pp. 221–224.
Al-Karaki, G.N., Growth of Mycorrhizal Tomato and Mineral Acquisition under Salt Stress, Mycorrhiza, 2000, vol. 10, pp. 51–54.
Abbaspour, H., Fallahyan, F., and Fahimi, H., Effects of Endomycorrhizal Fungi and Salt Stress on Nutrient Acquisition and Growth of Pistacia vera, Pakistan J. Biol. Sci., 2005, vol. 8, pp. 1006–1010.
Al-Karaki, G.N. and Al-Raddad, A., Effect of Arbuscular Mycorrhizal Fungi and Drought Stress on Growth and Nutrient Uptake of Two Wheat Genotypes Differing in Drought Resistance, Mycorrhiza, 1997, vol. 7, pp. 83–88.
Ruiz-Lozano, J.M. and Azcon, R., Hyphal Contribution to Water Uptake in Mycorrhizal Plants as Affected by the Fungal Species and Water Status, Physiol. Plant., 1995, vol. 95, pp. 472–478.
Poss, J.A., Pond, E., Menge, J.A., and Harrell, W.M., Effect of Salinity on Mycorrhizal Onion and Tomato in Soil with and without Additional Phosphate, Plant Soil, 1985, vol. 88, pp. 307–319.
Mercy, M.A., Shivanshanker, G., and Bagyaraj, D.J., Mycorrhizal Colonization in Cowpea Is Host Dependent and Heritable, Plant Soil, 1990, vol. 121, pp. 292–294.
Abousalim, A. and Mantel, S.H., Micrografting of Pistachio (Pistacia vera L. cv. Mateur), Plant Cell, Tissue Organ Cult., 1992, vol. 29, pp. 231–234.
Phillips, J. and Hayman, D., Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection, Trans. Br. Mycol. Soc., 1970, vol. 55, pp. 158–161.
Bierman, B. and Linderman, R., Quantifying Vesicular Arbuscular Mycorrhizae: Proposed Method towards Standardization, New Phytol., 1981, vol. 87, pp. 63–67.
Watanabe, F.S. and Olsen, S., Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extract for Soil, Soil Sci., 1965, vol. 21, pp. 677–678.
Ryan, J., Garabet, S., Harmsen, K., and Rashid, A., A Soil and Plant Analysis Manual Adapted for the West Asia and North Africa Region, ICARDA, Allepo, Syria, 1996.
Al-Karaki, G.N., Growth and Mineral Acquisition by Mycorrhizal Tomato Grown under Salt Stress, Mycorrhiza, 2000, vol. 10, pp. 51–54.
Diets, K.J. and Foyer, C., The Relationship between Phosphate and Photosynthesis in Leaves. Reversibility of the Effects of Phosphate Deficiency on Photosynthesis, Planta, 1986, vol. 167, pp. 376–381.
Sander, F.E. and Tinker, P.B., Phosphate Flow into Mycorrhizal Roots, Pest. Sci., 1973, vol. 4, pp. 385–395.
Joner, E.J., Ravnskov, S., and Jakobsen, I., Arbuscular Mycorrhizal Phosphate Transport under Monoxenic Condition using Radiolabeled Inorganic and Organic Phosphate, Biotechnol. Lett., 2000, vol. 22, pp. 1705–1708.
Al-Karaki, G.N., Barley Response to Salt Stress at Varied Phosphorus, J. Plant Nutr., 1997, vol. 20, pp. 1635–1643.
Jackobsen, I., Abbott, L.K., and Robson, A.D., External Hyphae of Vesicular-Arbuscular Mycorrhizal Fungi Associated with Trifolium subterraneum L.: 1. Spread of Hyphae and Phosphorus in Flow into Roots, New Phytol., 1992, vol. 120, pp. 371–380.
Burkert, B. and Robson, A.D., Zn Uptake in Subterranean Clover (Trifolium subterraneum L.) by Three Vesicular Arbuscular Mycorrhizal Fungi in a Root Free Sandy Soil, Soil Biol. Biochem., 1994, vol. 26, pp. 1117–1124.
Al-Karaki, G.N., Hammad, R., and Rusan, M., Response of Two Tomato Cultivars Differing in Salt Tolerance to Inoculation with Mycorrhizal Fungi under Salt Stress, Mycorrhiza, 2001, vol. 11, pp. 43–47.
Jarstfer, A.G., Farmer, P., and Sylvia, D.M., Tissue Magnesium and Calcium Affect Arbuscular Mycorrhizal Development and Fungal Reproduction, Mycorrhiza, 1998, vol. 7, pp. 237–242.
Munns, R., Physiological Processes Limiting Plant Growth in Saline Soils: Some Dogmas and Hypothesis, Plant Cell Environ., 1993, vol. 16, pp. 15–24.
Tawfic, M., Muhsin, T.M, and Zwiazek, J.J., Colonization with Hebeloma crustuliniforme Increases Water Conductance and Limits Shoot Sodium Uptake in White Spruce (Picea glauca) Seedlings, Plant Soil, 2002, vol. 238, pp. 217–225.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Fiziologiya Rastenii, 2010, Vol. 57, No.4, pp. 564–570.
This text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Abbaspour, H. Investigation of the effects of Vesicular Arbuscular Mycorrhiza on mineral nutrition and growth of Carthamus tinctorius under salt stress conditions. Russ J Plant Physiol 57, 526–531 (2010). https://doi.org/10.1134/S1021443710040102
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1021443710040102