Abstract
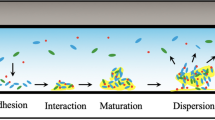
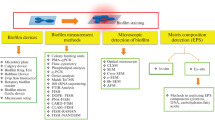
The structure, composition, and developmental patterns of multi-species biofilms are analyzed, as are the mechanisms of interaction of their microbial components. The main methodological approaches used for analysis of multi-species biofilms, including omics technologies, are characterized. Environmental communities (cyanobacterial mats and methanotrophic communities), as well as typical multi-species communities of medical importance (oral cavity, skin, and intestinal microbiomes), are described. A special section deals with the role of multi-species biofilms in such biotechnological processes as wastewater treatment, heavy metal removal, corrosion control, and environmental bioremediation.
Similar content being viewed by others
References
Abram, F., Systems-based approaches to unravel multi-species microbial community functioning, Comput. Structur. Biotechnol. J., 2015, vol. 13, pp. 24–32.
Adam, B., Baillie, G.S., and Douglas, L.J., Mixed species biofilms of Candida albicans and Staphylococcus epidermidis, J. Med. Microbiol., 2002, vol. 51, pp. 344–349.
Ahmad, F., Babalola, O.O., and Tak, H.I., Potential of MALDI-TOF mass spectrometry as a rapid detection technique in plant pathology: identification of plant-associated microorganisms, Anal. Bioanal. Chem., 2012. doi: 10.1007/s00216-012-6091-7
Al-Bader, D., Kansour, M., Rayan, R., and Radwan, S.S., Biofilm comprising phototrophic, diazotrophic, and hydrocarbon-utilizing bacteria: a promising consortium in the bioremediation of aquatic hydrocarbon pollutants, Environ. Sci. Pollut. Res., 2012, vol. 20, pp. 3252–3262.
Al-Mailem, D.M., Kansour, M.K., and Radwan, S.S., Hydrocarbonoclastic biofilms based on sewage microorganisms and their application in hydrocarbon removal in liquid wastes, Can. J. Microbiol., 2014, vol. 60, pp. 477–486.
Ali, H., Greco-Stewart, V.S., Jacobs, M.R., Yomtovian, R.A., Rood, I.G.H., de Korte, D., and Ramirez-Arcos, S.M., Characterization of the growth dynamics and biofilm formation of Staphylococcus epidermidis strains isolated from contaminated platelet units, J. Med. Microbiol., 2014, vol. 63, pp. 884–891.
Almstrand, R., Persson, F., Daims, H., Ekenberg, M., Christensson, M., Wilén, B.-M., Sörensson, F., and Hermansson, M., Three-dimensional stratification of bacterial biofilm populations in a moving bed biofilm reactor for nitritation-anammox, Int. J. Mol. Sci., 2014, vol. 15, pp. 2191–2206.
Angeletti, S., Dicuonzo, G., Avola, A., Crea, F., Dedej, E., Vailati, F., Farina, C., and De Florio, L., Viridans group streptococci clinical isolates: MALDI-TOF mass spectrometry versus gene sequence-based identification, PLoS One, 2015. doi: 10.1371/journalpone.0120502
Ansari, M.J., Al-Ghamdi, A., Usmani, S., Al-Waili, N.S., Sharma, D., Nuru, A., and Al-Attal, Y., Effect of jujube honey on Candida albicans growth and biofilm formation, Arch. Med. Res., 2013, vol. 44, pp. 352–360.
Arps, P.J., Earthman, J.C., Xu, L., Syrett, B.C., Green, R., Wood, T., and Mansfield, F.B., Field evaluation of corrosion control using regenerative biofilms (CCURB), Conf. Paper. Corrosion 2003. 16–20 March, San Diego, California, NACE International. Document ID: NACE-03714.
Asahi, Y., Miura, J., Tsuda, T., Kuwabata, S., Tsunashima, K., Noiri, Y., Sakata, T., Ebisu, S., and Hayashi, M. Simple observation of Streptococcus mutans biofilm by scanning electron microscopy using ionic liquids, AMB Express, 2015, vol. 5, no. 6. http://wwwncbinlmnihgov/pmc/articles/PMC4305086
Audrain, B., Farag, M.A., Ryu, C.-M., and Ghigo, J.-M., Role of bacterial volatile compounds in bacterial biology, FEMS Microbiol. Rev., 2015, vol. 39, pp. 222–233.
Azevedo, N.F., Lopes, S.P., Keevil, C.W., Pereira, M.O., and Vieira, M.J. Time to “go large” on biofilm research: advantages of an omics approach, Biotechnol. Lett., 2009, vol. 31, pp. 477–485.
Baum, M.M., Kainovic’, A., O’Keeffe, T., Pandita, R., McDonald, K., Wu, S., and Webster, P. Characterization of structures in biofilms formed by a Pseudomonas fluorescens isolated from soil, BMC Microbiol., 2009, vol. 9, pp. 1–13. http://wwwbiomedcentralcom/1471-2180/9/103
Beaussart, A., Herman, P., El-Kirat-Chatel, S., Lipke, P.N., Kucharíková, S., Van Dijck, P., and Dufrêne, Y.F., Single-cell force spectroscopy of the medically-important Staphyloccocus epidermidis–Candida albicans interaction, Nanoscale, 2013. 5(22). doi: 10.1039/c3nr03272h
Beyenal, H. and Babauta, J.T., Microscale gradients and their role in electron-transfer mechanisms in biofilms, Biochem. Soc. Trans., 2012, vol. 40, pp. 1315–1318.
Bhattacharyya, S., Gupta, P., Banerjee, G., Jain, A., and Singh, M., Inhibition of biofilm formation and lipase in Candida albicans by culture filtrate of Staphylococcus epidermidis in vitro, Int. J. Appl. Basic Med. Res., 2014, vol. 4, pp. 27–30.
Bochner, B.R., New technologies to assess genotype–phenotype relationships, Nature Rev. Genet., 2003, vol. 4, pp. 309–314.
Bolhuis, H., Fillinger, L., and Stal, L.J., Coastal microbial mat diversity along a natural salinity gradient, PLoS One, 2013. 8(5):e63166. doi: 10.1371/journalpone.0063166
Bolhuis, H., Cretoiu, M.S., and Stal, L.J., Molecular ecology of microbial mats, FEMS Microbiol. Ecol., 2014, vol. 90, pp. 335–350.
Botchkova, E.A., Litti, Yu. V., Kuznetsov, B.B., and Nozhevnikova, A.N., Microbial biofilms formed in a laboratory-scale anammox bioreactor with flexible bruch carrier, J. Biomater. Nanobiotechnol., 2014, vol. 5, pp. 76–82.
Botchkova, E.A., Plakunov, V.K., and Nozhevnikova, A.N., Dynamics of biofilm formation on microscopic slides sbmerged in an anammox bioreactor, Microbiology (Moscow), 2015, vol. 84., no. 3, pp. 456–460.
Breugelmans, P., Barken, K.B., Tolker-Nielsen, T., Hofkens, J., Dejonghe, W., and Springael, D., Architecture and spatial organization in a triple-species bacterial biofilm synergistically degrading the phenylurea herbicide linuron, FEMS Microbiol. Ecol., 2008, vol. 64, pp. 271–282.
Brileya, K.A., Camilleri, L.B., and Fields, M.W., 3D-Fluorescence in situ hybridization of intact, anaerobic biofilm, Methods Mol. Biol., Sun, L. and Shou, W., Eds., New York: Springer, 2014, vol. 1151, pp. 189–197.
Burmølle, M., Ren, D., Bjarnsholt, T., and Sørensen, S.J., Interactions in multi-species biofilms: do they actually matter?, Trends Microbiol., 2014, vol. 22, pp. 84–91.
Burow, L.C., Woebken, D., Marshall, I.P.G., Lindquist, E.A, Bebout, B.M., Prufert-Bebout, L., Hoehler, T.M., Tringe, S.G., Pett-Ridge, J., Weber, P.K., Spormann, A.M., and Singer, S.W., Anoxic carbon flux in photosynthetic microbial mats as revealed by metatranscriptomics, ISME J., 2013, vol. 7, pp. 817–829.
Cao, B., Shi, L., Brown, R.N., Xiong, Y., Fredrickson, J.K., Romine, M.F., Marshall, M.J., Lipton, M.S., and Beyenal, H., Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics, Environ. Microbiol., 2011, vol. 13, pp. 1018–1031.
Chae, K.-J., Kim, S.-M., Oh, S.-E., Ren, X., Lee, J., and Kim, I.S., Spatial distribution and viability of nitrifying, denitrifying and ANAMMOX bacteria in biolms of sponge media retrieved from a full-scale biological nutrient removal plant, Bioproces. Biosyst. Eng., 2012, vol. 35, pp. 1157–1165.
Chai, L., Huang, S., Yang, Z., Peng, B., and Huang, Y., Cr(VI) remediation by indigenous bacteria in soils contaminated by chromium-containing slag, J. Hazard Mater., 2009, vol. 167, pp. 516–522.
Chalupova, J., Raus, M., Sedlarova, M., and Sebala, M., Identification of fungal microorganisms by MALDI-TOF mass spectrometry, Biotechnol. Adv., 2014, vol. 32, pp. 230–241.
Chandra, J., Mukherjee, P.K., and Ghannoum, M.A., In vitro growth and analysis of Candida biofilms, Nat. Protoc., 2008, vol. 3, pp. 1909–1924.
Chao, Y. and Zhang, T., Surface-enhanced Raman scattering (SERS) revealing chemical variation during biofilm formation: from initial attachment to mature biofilm, Anal. Bioanal. Chem., 2012, vol. 404, pp. 1465–1475.
Chao, Q.-T., Lee, T.-F., Teng, S.-H., Peng, L.-Y., Chen, P.H., Teng, L.-J., and Hsueh, P.-R., Comparison of the accuracy of two conventional phenotypic methods and two MALDI-TOF MS systems with that of DNA sequencing analysis for correctly identifying clinically encountered yeasts, PLoS One, 2014. 9(10): e109376. doi: 10.1371/journalpone.0109376
Cho, S., Takahashi, Y., Fujii, N., Yamada, Y., Satoh, H., and Okabe, S. Nitrogen removal performance and microbial community analysis of an anaerobic up-flow granular bed anammox reactor, Chemosphere, 2010, vol. 78, pp. 1129–1135.
Christensen, G.J. and Brüggemann, H., Bacterial skin commensals and their role as host guardians, Benef. Microbes, 2014, vol. 5, pp. 201–215.
Cole, J.K., Hutchison, J.R., Renslow, R.S., Kim, Y.-M., Chrisler, W.B., Engelmann, H.E., Dohnalkova, A.C., Hu, D., Metz, T.O., Fredrickson, J.K., and Lindemann, S.R., Phototrophic biofilm assembly in microbial-mat-derived unicyanobacterial consortia: model systems for the study of autotroph-heterotroph interactions, Front. Microbiol., 2014, vol. 5. A. 109. wwwfrontiersinorg
Conlon, B.P., Rowe, S.E., and Lewis, K., Persister cells in biofilm associated infections, Adv. Exp. Med. Biol., 2015, vol. 831, pp. 1–9.
Coppenhagen-Glazer, S., Sol, A., Abed, J., Naor, R., Zhang, X., Han, Y.W, and Bachrach, G., Fap2 of Fusobacterium nucleatum is a galactose-inhibitable adhesin involved in coaggregation, cell adhesion, and preterm birth, Infect. Immun., 2015, vol. 83, pp. 1104–1113.
Costerton, J.W., Cheng, K.J., Geesey, G.G., Ladd, T.I., Nickel, J.C., Dasgupta, M., and Marrie, T.J., Bacterial biofilms in nature and disease, Annu. Rev. Microbiol., 1987, vol. 41, pp. 435–464.
Cote, C., Rosas, O., and Basseguy, R., Geobacter sulfurreducens: an iron reducing bacterium that can protect carbon steel against corrosion?, Corrosion Sci., 2015, vol. 94, pp. 104–113.
Daims, H. and Wagner, M., In situ techniques and digital image analysis methods for quantifying spatial localization patterns of nitrifiers and other microorganisms in biofilm and flocs, in Methods Enzymol., Abelson, J.N. and Simon, M.L., Eds., San Diego: Elsevier, 2011, vol. 496, pp. 185–215.
Darwish, S.F. and Asfour, H.A.E., Investigation of biofilm forming ability in Staphylococci causing bovine mastitis using phenotypic and genotypic assays, Sci. World J., 2013. Article ID378492. 9 p. http://dxdoiorg/10.1155/2013/378492
De Muynck, W., Debrouwer, D., De Belie, N., and Verstraete, W., Bacterial carbonate precipitation improves the durability of cementitious materials, Cem. Concr. Res., 2008, vol. 38, pp. 1005–1014.
Diaz, P.I., Strausbaugh, L.D., and DongariBagtzoglou, A., Fungal-bacterial interactions and their relevance to oral health: linking the clinic and the bench, Front. Cell. Infect. Microbiol. 2014, vol. 4. A. 101. wwwfrontiersinorg
Dillon, J.G., Miller, S., Bebout, B., Hullar, M., Pinel, N., and Stahl, D.A., Spatial and temporal variability in a stratified hypersaline microbial mat community, FEMS Microbiol. Ecol., 2009, vol. 68, pp. 46–58.
Dufreêne, Y.F., Sticky microbes: forces in microbial cell adhesion, Trends Microbiol., 2015. 1–7. http://dx. doiorg/10.1016/jtim.2015.01.011
Dupuy, A.K., David, M.S., Li, L., Heider, T.N., Peterson, J.D., Montano, E.A., Dongari-Bagtzoglou, A., Diaz, P.I., and Strausbaugh, L.D., Redefining the human oral mycobiome with improved practices in ampliconbased taxonomy: discovery of Malassezia as a prominent commensal, PLoS One, 2014. 9:e90899. doi: 10.1371/journalpone.0090899
Elson, C.O. and Alexander, K.L., Host-microbiota interactions in the intestine, Digest. Diseas., 2015, vol. 33, pp. 131–136.
Everroad, R.C., Otaki, H., Matsuura, K., and Haruta, S., Diversification of bacterial community composition along a temperature gradient at a thermal spring, Microb. Environ., 2012, vol. 27, pp. 374–381.
Famdale, R.W., Sayers, C.A., and Barrett, A.J., A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures, Connect. Tissue Res., 1982, vol. 9, pp. 247–248.
Faust, K. and Raes, J., Microbial interactions: from networks to models, Nature Revs. Microbiol., 2012, vol. 10, pp. 538–550.
Feazel, L.M., Baumgartner, L.K., Peterson, K.L., Frank, D.N., Harris, J.K., and Pace, N.R., Opportunistic pathogens enriched in showerhead biofilms, Proc. Natl. Acad. Sci. U. S. A., 2009, vol. 106, pp. 16393–16399.
Fernandes, I., Vazques-Padin, J.R., Mosquera-Corral, A., Campos, J.L., and Mendes, R., Biofilm and granular systems to improve Anammox biomass retention, Biochem. Eng. J., 2008, vol. 42, pp. 308–313.
Findley, K., Oh, J., Yang, J., Conlan, S., Deming, C., Meyer, J.A., Schoenfeld, D., Nomicos, E., and Park, M., Topographic diversity of fungal and bacterial communities in human skin, Nature, 2013, vol. 498, pp. 367–370.
Finkenstadt, V.L., Cote, G.L., and Willett J.L., Corrosion protection of low-carbon steel using exopolysaccharide coatings from Leuconostoc mesenteroides, Biotechnol. Lett., 2011, vol. 33, pp. 1093–1100.
Fish, K.E., Collins, R., Green, N.H., Sharpe, R.L., Douterelo, I., Osborn, A.M., and Boxall, J.B., Characterisation of the physical composition and microbial community structure of biofilms within a model full-scale drinking water distribution system, PLoS One, 2015. doi: 10.1371/journalpone.0115824 February 23.
Franzosa, E.A., Morgan, X.C., Segata, N., Waldron, L., Reyes, J., Earl, A.M., Giannoukos, G., Boylan, M.R., Ciulla, D., Gevers, D., Izard, J., Garrett, W.S., Chan, A.T., and Huttenhower, C., Relating the metatranscriptome and metagenome of the human gut, Proc. Natl. Acad. Sci. U. S. A., 2014, vol. 111, pp. 2329–2338.
Frols, S., Archaeal biofilms: widespread and complex, Biochem. Soc. Trans., 2013, vol. 41, pp. 393–398.
Fuchsman, C.A., Staley, J.T., Oakley, B.B., Kirkpatrick, J.B., and Murray, J.W., Free-living and aggregate-associated Planctomycetes in the Black Sea, FEMS Microbiol. Ecol., 2012, vol. 80, pp. 402–416.
Furuhata, K., Ishizaki, N., and Fukuyama, M. Characterization of heterotrophic bacteria isolated from the biofilm of a kitchen sink, Biocontrol Sci., 2010, vol. 358, pp. 91–118.
Gannesen, A.V., Zhurina, M.V., Veselova, M.A., Khmel’, I.A., and Plakunov, V.K., Regulation of biofilm formation by Pseudomonas chlororaphis in an in vitro system, Microbiology (Moscow), 2015, vol. 84, no. 3, pp. 319–327.
Giacomoni, P.U., Mammone, T., and Teri, M., Genderlinked differences in human skin, J. Dermatol. Sci., 2009, vol. 55, pp. 44–149.
Gieseke, A., Arnz, P., Amann, R., and Schramm, A., Simultaneous P and N removal in a sequencing batch biofilm reactor: insights from reactorand microscale investigations, Water Res., 2002, vol. 36, pp. 501–509.
Hall-Stoodley, L., Costerton, J.W., and Stoodley, P., Bacterial biofilms: from the natural environment to infectious diseases, Nature Rev. Microbiol., 2004, vol. 2, pp. 95–108.
Herath, H.M.L.I., Upam, A., Rajapakshab, U., Vithanageb, M., and Seneviratnea, G., Developed fungal–bacterial biofilms as a novel tool for bioremoval of hexavalent chromium from wastewater, Chem. Ecol., 2014, vol. 30, pp. 418–427.
Hidalgo, G., Burns, A., Herz, E., Hay, A.G., Houston, P.L., Wiesner, U., and Lion, L.W., Functional tomographic fluorescence imaging of pH microenvironments in microbial biofilms by use of silica nanoparticle sensors, Appl. Environ. Microbiol., 2009, vol. 75, pp. 7426–7435.
Hogardt, M. and Heesemann, J., Microevolution of Pseudomonas aeruginosa to a chronic pathogen of the cystic fibrosis lung, Curr. Top. Microbiol. Immunol., 2013, vol. 358, pp. 91–118.
Horz, H.-P., Archaeal lineages within the human microbiome: absent, rare or elusive?, Life, 2015, vol. 5, pp. 1333–1345.
Hurse, T.J. and Keller, J., Reconsidering the use of photosynthetic bacteria for removal of sulfide from wastewater, Biotechnol. Bioeng., 2004, vol. 85, pp. 47–55.
Hsu, Y.-M.S. and Burnham, C.-A.D., MALDI-TOF MSidentification of anaerobic bacteria: assessment of pre-analytical variables and specimen preparation techniques, Diagnost. Microbiol. Infect. Dis., 2014. doi: 10.1016/jdiagmicrobio.2014.02.007
Hsueh, P.-R., Lee, T.F., Du, S.-H., Teng, S.-H., Liao, C.-H., Sheng, W.-H., and Teng, L.-J., Bruker biotyper matrixassisted laser desorption ionization–time of flight mass spectrometry system for identification of Nocardia, Rhodococcus, Kocuria, Gordonia, Tsukamurella, and Listeria species, J. Clin. Microbiol., 2014, vol. 52, pp. 2371–2379.
Huang, R., Li, M., and Gregory, R.L., Bacterial interactions in dental biofilm, Virulence, 2011, vol. 2, pp. 435–444.
Iwase, T., Uehara, Y., Shinji, H., Tajima, A., Seo, H., Takada, K., Agata, T., and Mizunoe, Y., Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization, Nature, 2010, vol. 465, pp. 346–349.
Jackson, V.A., Paulse, A.N., Bester, A.A., Neethling, J.H., Khana, S., and Khanb, W., Bioremediation of metal contamination in the Plankenburg River, Western Cape, South Africa, Int. Biodeterior. Biodegr., 2009, vol. 63, pp. 559–568.
Jakubovics, N.S., Yassin, S.A., and Rickard, A.H., Community interactions of oral streptococci, Adv. Appl. Microbiol., 2014, vol. 87, pp. 43–110.
Janssen, A.W.F. and Kersten, S., The role of the gut microbiota in metabolic health, FASEB. J., 2015. Apr. 28. pii: fj.14-269514. wwwfasebjorg
Jayaraman, A., Ornek, D., Duarte, D.A., Lee, C.-C., Mansfeld, F.B., and Wood, T.K., Axenic aerobic biofilms inhibit corrosion of copper and aluminum, Appl. Microbiol., Biotechnol. 1999, vol. 52, pp. 787–790.
Jefferson, K.K., What drives bacteria to produce a biofilm?, FEMS Microbiol. Lett., 2004, vol. 236, pp. 163–173.
Jensen, A.B. and Webb, C., Treatment of H2S-containing gases: A review of microbiological alternatives, Enz. Microbial Technol., 1995, vol. 17, pp. 2–10.
Ivleva, N.P., Wagner, M., Szkola, A., Horn, H., Niessner, R., and Haisch, C., Label-free in situ SERS imaging of biofilms, J. Phys. Chem., 2010, vol. 114, pp. 10184–10194.
Kamaeva, A.A., Vasilchenko, A.S., and Deryabin, D.G., Atomic force microscopy reveals a morphological differentiation of Chromobacterium violaceum cells associated with biofilm development and directed by N-hexanoyl-Lhomoserine lactone, PLoS One, 2014. 9(8): e103741. doi:10.1371/journalpone.0103741
Kim, H.-S. and Park, H.-D., Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14, PLoS One, 2013. 8(9): e76106. doi: 10.1371/journal. pone.0076106
Kim, T.G., Yi, T., Lee, E.-H., Ryu, H.W., and Cho, K.-S., Characterization of a methane-oxidizing biofilm using microarray, and confocal microscopy with image and geostatic analyses, Appl. Microbiol. Biotechnol., 2012, vol. 95, pp. 1051–1059.
Kindaichi, T., Tsushima, I., Ogasawara, Y., Shimokawa, M., Ozaki, N., Satoh, H., and Okabe, S., In situ activity and spatial organization of anaerobic ammonium oxidizing (anammox) bacteria in biofilms, Appl. Environ. Microbiol., 2007, vol. 73, pp. 4931–4939.
Kindaichi, T., Yuri, S., Ozaki, N., and Ohashi, A., Ecophysiological role and function of uncultured Chlroflexi in an anammox reactor, Water Sci. Technol., 2012, vol. 66, pp. 2556–2561.
Kip, N. and van Veen, J.A., The dual role of microbes in corrosion, ISME J., 2015, vol. 9, pp. 542–555.
Klatt, C.G., Liu, Z., Ludwig, M., Kuhl, M., Jensen, S.I., Bryant, D.A., and Ward, D.M., Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring, ISME J., 2013a, vol. 7, pp. 1775–1789.
Klatt, C.G., Inskeep, W.P., Herrgard, M.J., Jay, Z.J., Rusch, D.B., Tringe, S.G., Parenteau, M.N., Ward, D.M., Boomer, S.M., Bryant, D.A., and Miller, S.R., Community structure and function of high-temperature chlorophototrophic microbial mats inhabiting diverse geothermal environments, Front. Microbiol., 2013b, vol. 4, pp. 1–23.
Kolenbrander, P.E., multi-species communities: interspecies interactions influence growth on saliva as sole nutritional source, Int. J. Oral. Sci., 2011, vol. 3, pp. 49–54.
Kostka, J.E. and Green, S.J., Microorganisms and processes linked to uranium reduction and immobilization, in Microbial Metal and Metalloid Metabolism: Advances and Applications, Stolz, J.F. and Oremland, R.S., Eds., Washington: ASM, 2011, pp. 117–138.
Kratochvil, D. and Volesky, B., Biosorption of Cu from ferruginous wastewater by algal biomass, Water Res., 1998, vol. 32, pp. 2760–2768.
Larsen, P., Olesen, B.H., Nielsen, P.H., and Nielsen, J.L., Quantification of lipids and protein in thin biofilms by fluorescence staining, Biofouling, 2008, vol. 24, pp. 241–250.
Li, X.-R., Du, B., Fu, H.-X., Wang, R.-F., Shi, J.-H., Wang, Y., Jetten, M.S.M., and Quan, Z.-X., The bacterial diversity in ananaerobic ammonium-oxidizing (anammox) reactor community, Syst. Appl. Microbiol., 2009, vol. 32, pp. 278–289.
Li, X.-R., Xiao, Y., Liao, D., Zheng, W., Yi, T., Yang, Q., and Zeng, G., Granulation of simultaneous partial nitrification and anammox biomass in one single SBR system, Appl. Biochem. Biotechnol., 2011, vol. 163, pp. 1053–1065.
Liehr, S.K., Chen, H.J. and Lin, S.H., Metals removal by algal biofilms, Water Sci. Technol., 1994, vol. 30, pp. 59–68.
Liu, C., Yamamoto, Y., Nishiyama, T., Fujii, T., and Furukawa, K., Effect of salt concentration in anammox treatment using non woven biomass carrier, J. Biosci. Bioeng., 2009, vol. 107, pp. 519–523.
Llirós, M., Gajua, N., de Oteyza, T.G., Grimaltb, J.O., Estevea, I., and Martínez-Alonso, M., Microcosm experiments of oil degradation by microbial mats. II. The changes in microbial species, Sci. Total Environ., 2008, vol. 393, pp. 39–49.
Loozen, G., Ozcelik, O., Boon, N., De Mol, A., Schoen, C., Quirynen, M., and Teughels, W., Inter-bacterial correlations in subgingival biofilms: a largescale survey, J. Clin. Periodontol., 2014, vol. 41, pp. 1–10.
López-López, A., Richter M., Peña, A., Tamames, J., and Rosselló-Móra, R., New insights into the archaeal diversity of a hypersaline microbial mat obtained by a metagenomic approach, Syst. Appl. Microbiol., 2013, vol. 36, pp. 205–214.
Lourenco, A., Ferreira, A., Veiga, N., Machado, I., Pereira, M.O., and Azevedo N.F., BiofOmics: a web platform for the systematic and standardized collection of highthroughput biofilm data, PLoS One, 2012. 7(6): e39960. doi:10.1371/journalpone.0039960
Magnúsdóttir, S., Ravcheev, D., de Crécy-Lagard, V., and Thiele, I., Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes, Front. Genet., 2015, vol. 6. A. 14. wwwfrontiersinorg
Mahajan, A., Singh, B., Kashyap, D., Kumar, A., and Mahajan, P., Interspecies communication and periodontal disease, Sci. World J., 2013. doi: 10.1155/2013/765434
Mang, T.S., Tayal, D.P., and Baier, R., Photodynamic therapy as an alternative treatment for disinfection of bacteria in oral biofilms, Laser. Surg. Med., 2012, vol. 44, pp. 588–596.
Mart’yanov, S.V., Zhurina, M.V., El’Registan, G.I., and Plakunov, V.K., Activation of formation of bacterial biofilms by azithromycin and prevention of this effect, Microbiology (Moscow), 2014, vol. 83, no. 6, pp. 723–731.
Mashima, I. and Nakasawa, F. The interaction between Streptococcus spp. and Veillonella tobetsuens on the early stages of oral biofilm formation, J. Bacteriol., 2015. doi: 10.1128/JB.02512-1.
McLean, J.S., Advancements toward a systems level understanding of the human oral microbiome, Front. Cell. Infect. Microbiol., 2014, vol. 4. A. 98. wwwfrontiersinorg
Melander, R.J. and Melander, C., Innovative strategies for combating biofilm-based infections, Adv. Exp. Med. Biol., 2015, vol. 831, pp. 69–91.
Metayer-Levrel, G.L., Castanier, L.S., Orial, G., Loubière, J.-F., and Perthuisot, J.-P., Applications of bacterial carbonatogenesis to the protection and regeneration of limestones in buildings and historic patrimony, Sediment. Geol., 1999, vol. 126, pp. 25–34.
Molina, M.A., Ramos, J.-L., and Espinosa-Urgel, M., Plant-associated biofilms, Revs. Environ. Sci. BioTechnol., 2003, vol. 2, pp. 99–108.
Momeni, B., Brileya, K.A., Fields, M.W., and Shou, W., Strong inter-population cooperation leads to partner intermixing in microbial communities, Elife, 2013. Jan. 22, 2:e00230. doi: 10.7554/eLife.00230
Moran, N.A., McLaughlin, H.J., and Sorek, R., The dynamics and time scale of ongoing genomic erosion in symbiotic bacteria, Science, 2009, vol. 323, pp. 379–382.
Morris, J.J., Lenski, R.E., and Zinser, E.R., The black queen hypothesis: evolution of dependencies through adaptive gene loss, mBio, 2012. 3(2):e00036–12. doi: 10.1128/mBio.00036-12
Netuschil, L., Auschill, T.M., Sculean, A., and Arweiler, N.B., Confusion over live/dead stainings for the detection of vital microorganisms in oral biofilms–which stain is suitable?, BMC Oral Health, 2014, vol. 14, pp. 2–12.
Neu, T.R. and Lawrence, J.R., Investigation of microbial biofilm structure by laser scanning microscopy, Adv. Biochem. Eng. Biotechnol., 2014, vol. 146, pp. 1–51.
Neu, T.R. and Lawrence, J.R., Innovative techniques, sensors, and approaches for imaging biofilms at different scales, Trends Microbiol., 2015, vol. 23, pp. 233–242.
Ni, S.-Q., Sun, N., Yang, H., Zhang, J., and Ngo, H.H., Distribution of extracellular polymeric substances in anammox granules and their important roles during anammox granulation, Biochem. Engineer. J., 2015. http:// dxdoiorg/10.1016/jbej.2015.05.014
Nikolaev, Yu.A. and Plakunov, V.K., Biofilm—“City of microbes” or an analogue of multicellular organisms?, Microbiology (Moscow) 2007, vol. 76, no. 2, pp. 125–138.
Nosyk, O., Haseborg, E., Metzger, U., and Frimmel, F.H., A standardized pre-treatment method of biofilm flocs for fluorescence microscopic characterization, J. Microbiol. Methods, 2008, vol. 75, pp. 449–456.
Oh, J., Byrd, A.L., Deming, C., and Conlan, S., NISC comparative sequencing program, Kong, H.H., and Segre, J.A. Biogeography and individuality shape function in the human skin metagenome, Nature, 2014, vol. 514, pp. 59–64.
Okabe, S., Ito, T., and Satoh, H. Sulfate-reducing bacterial community structure and their contribution to carbon mineralization in a wastewater biofilm growing under microaerophilic conditions, Appl. Microbiol. Biotechnol., 2003, vol. 63, pp. 322–334.
Okabe, S., Kindaichi, T., and Ito, T., Fate of 14C-labeled microbial products derived from nitrifying bacteria in autotrophic nitrifying biofilms, Appl. Environ. Microbiol., 2005, vol. 71, pp. 3987–3994.
Orell, A., Frols, S., and Albers, S.-V., Archaeal biofilms: the great unexplored, Annu. Rev. Microbiol., 2013, vol. 67, pp. 337–354.
Otaki, H., Everroad, R.C., Matsuura, K., and Haruta, S., Production and consumption of hydrogen in hot spring microbial mats dominated by a filamentous anoxygenic photosynthetic bacterium, Microb. Environ., 2012, vol. 27, pp. 293–299.
Oumeraci, T., Jensen, V., Talbot, S.R., Hofmann, W., Kostrzewa, M., Schlegelberger, B., von Neuhoff, N., and Häussler, S. Comprehensive MALDI-TOF biotyping of the nonredundant harvard Pseudomonas aeruginosa PA14 transposon insertion mutant library, PLoS One, 2015. doi: 10.1371/journalpone.0117144
Ovchinnikova, E.S., Krom, B.P., Busscher, H.J., and van der Mei, H.C., Evaluation of adhesion forces of Staphylococcus aureus along the length of Candida albicans hyphae, BMC Microbiol., 2012. 12:281. http://wwwbiomedcentralcom/1471-2180/12/281
Panda, A., Kurapati, S., Samantaray, J.C., Srinivasan, A., and Khalil, S., MALDI-TOF mass spectrometry proteomic based identifiation of clinical bacterial isolates, Indian J. Med. Res., 2014, vol. 140, pp. 770–777.
Pantanella, F., Berlutti, F., Passariello, C., Sarli, S., Morea, C., and Schippa, S., Violacein and biofilm production in Janthinobacterium lividum, J. Appl. Microbiol., 2007, vol. 102, pp. 992–999.
Pantanella, F., Valenti, P., Natalizi, T., Passeri, D., and Berlutti, F., Analytical techniques to study microbial biofim on abiotic surfaces: pros and cons of the main techniques currently in use, Annali di igiene, 2013, vol. 25, pp. 31–42.
Pastorella, G., Gazzola, G., Guadarrama, S., and Marsili, E., Biofilms: applications in bioremediation, in Microbial Biofilms—Current Research and Applications, Lear, G. and Lewis, G., Eds., Norfolk, UK: Caister Academic Press, 2012, pp. 73–98.
Pätzold, R., Keuntje, M., and Anders-von Ahlften, A., A new approach to non-destructive analysis of biofilms by confocal Raman microscopy, Anal. Bioanal. Chem., 2006, vol. 386, pp. 286–292.
Pätzold, R., Keuntje, M., Theophile, K., Müller, J., Mielcarek, E., Ngezahayo, A., and Anders-von Ahlften, A., In situ mapping of nitrifiers and anammox bacteria in microbial aggregates by means of confocal resonance Raman microscopy, J. Microbiol. Methods, 2008, vol. 72, pp. 241–248.
Peeters, E., Nelis, H.J., and Coenye, T., Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates, J. Microbiol. Methods, 2008, vol. 72, pp. 157–165.
Pérez-Rodríguez, G., Glez-Pena, D., Azevedo, N.F., Pereira, M.O., Fdez-Riverola, F., and Lourenco, A., Enabling systematic, harmonised and large-scale biofilms data computation: the biofilms experiment workbench, Comp. Meth. Progr. Biomed., 2015, vol. 118, pp. 309–321.
Persson, F., Sultana, R., Suarez, M., Hermansson, M., Plaza, E., and Wilen, B.-M., Structure and composition of biofilm communities in a moving bedbiofilm reactor for nitritation–anammox at low temperatures, Biores. Technol., 2014, vol. 154, pp. 267–273.
Plakunov, V.K., Strelkova, E.F., and Zhurina, M.V., Persistence and adaptive mutagenesis in biofilms, Microbiology (Moscow), 2010, vol. 79, no. 4, pp. 424–434.
Plyuta, V.A., Popova, A.A., Koksharova, O.A., and Khmel’, I.A., Effect of volatile organic compounds on Agrobacterium tumefaciens cells during biofilm formation and in mature biofilms, “Perspektivnye napravleniya fizikokhimicheskoi biologii i biotekhnologii” (Promising Directions in Physicochemical Biology and Biotechnology, Proc. 25th Int. Winter Youth Sci. School, Moscow), 2013, p. 104.
Potekhina, J.S., Sherisheva, N.G., Povetkina, L.P., Pospelov, A.P., Rakitina, T.A., Warnecke, F., and Gottschalk, G., Role of microorganisms in corrosion inhibition of metals in aquatic habitats, Appl. Microbiol. Biotechnol., 1999, vol. 52, pp. 639–646.
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K.S., Manichanh, C., Nielsen, T., Pons, N., Levenez, F., Yamada, T., Mende, D.R., Li, J., Xu, J., Li, S., Li, D., Cao, J., Wang, B., Liang, H., Zheng, H., Xie, Y., Tap, J., Lepage, P., Bertalan, M., Batto, J.M., Hansen, T., Le Paslier, D., Linneberg, A., Nielsen, H.B., Pelletier, E., Renault, P., Sicheritz-Ponten, T., Turner, K., Zhu, H., Yu, C., Jian, M., Zhou, Y., Li, Y., Zhang, X., Qin, N., Yang, H., Wang, J., Brunak, S., Dore, J., Guarner, F., Kristiansen, K., Pedersen, O., Parkhill, J., Weissenbach, J., Bork, P., and Ehrlich, S.D., A human gut microbial gene catalogue established by metagenomic sequencing, Nature, 2010, vol. 464, pp. 59–65.
Renslow, R.S., Babauta, J.T., Majors, P.D., and Beyenal, H., Diffusion in biofilms respirating in electrodes, Energy Environ. Sci., 2013, vol. 6, pp. 595–607.
Rickard, A.H., Gilbert, P., High, N.J., Kolenbrander, P.E., and Handley, P.S., Bacterial coaggregation: an integral process in the development of multi-species biofilms, Trends Microbiol., 2003, vol. 11, pp. 94–100.
Roberts, F.A. and Darveau, R.P., Beneficial bacteria of the periodontium, Periodontology, 2002, vol. 30, pp. 40–50.
Roberts, A.P., and Kreth, J. The impact of horizontal gene transfer on the adaptive ability of the human oral microbiome, Front. Cellul. Infect. Microbiol., 2014, vol. 4. A. 124. wwwfrontiersinorg
Robertson, C.E., Spear, J.R., Harris, J.K., and Pace, N.R., Diversity and stratification of archaea in a hypersaline microbial mat, Appl. Environ. Microbiol., 2009, vol. 75, pp. 1801–1810.
Roeselers, G., van Loosdrecht, M.C.M., and Muyze, G., Phototrophic biofilms and their potential applications, J. Appl. Phycol., 2008, vol. 20, pp. 227–235.
Rossi, F. and De Philippis, R., Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats, Life, 2015, vol. 5, pp. 1218–1238.
Rudrappa, T., Biedrzycki, M.L., and Bais, H.P., Causes and consequences of plant-associated biofilms, FEMS Microbiol. Ecol., 2008, vol. 64, pp. 153–166.
Sadykov, M.R., Zhang, B., Halouska, S., Nelson, J.L., Kreimer, L.W., Zhu, Y., Powers, R., and Somerville, G.A., Using NMR metabolomics to investigate tricarboxylic cid cycle dependent signal transduction in Staphylococcus epidermidis, J. Biol. Chem., 2010, vol. 285, pp. 36616–36624.
Sandoval-Carrasco, C.A., Ahuatzi-Chacón, D., GalíndezMayer, J., Ruiz-Ordaz, N., Juárez-Ramírez, C., and Martínez-Jerónimo, F., Biodegradation of a mixture of the herbicides ametryn, and 2,4-dichlorophenoxyacetic acid (2,4-D) in a compartmentalized biofilm reactor, Biores. Technol., 2013, vol. 145, pp. 33–36.
SanMiguel, A. and Grice, E.A., Interactions between host factors and the skin microbiome, Cell. Mol. Life Sci., 2014, vol. 72, pp. 1499–1515.
Satoh, H., Miura, Y., Tsushima, I., and Okabe, S., Layered structure of bacterial and archaeal communities and their in situ activities in anaerobic granules, Appl. Environ. Microbiol., 2007, vol. 73, pp. 7300–7307.
Schulthess, B., Bloemberg, G.V., Zbinden, R., Böttger, E.C.,and Hombach, M., Evaluation of the Bruker MALDI biotyper for identification of gram-positive rods: development of a diagnostic algorithm for the clinical laboratory, J. Clin. Microbiol., 2014, vol. 52, pp. 1089–1097.
Schopf, J.W., Solution to Darwin’s dilemma: discovery of the missing Precambrian record of life, Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, pp. 6947–6953.
Schumacher, G. and Sekoulov, I., Polishing of secondary effluent by an algal biofilm process, Water Sci. Technol., 2002, vol. 46, pp. 83–90.
Sergeev, V.N., Gerasimenko, L.M., and Zavarzin, G.A., The proterozoic history and present state of cyanobacteria, Microbiology (Moscow), 2002, vol. 71, no. 6, pp. 623–637.
Shoaie, S. and Nielsen, J., Elucidating the interactions between the human gut microbiota and its host through metabolic modeling, Front. Genet., 2014. 5: 86. doi: 10.3389/fgene.2014.00086
Simões, L.C., Simões, M., and Vieira, M.J., Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium, Appl. Environ. Microbiol., 2008, vol. 74, pp. 1259–1263.
Singh, R., Paul, D., and Jain, R.K., Biofilms: implications in bioremediation, Trends Microbiol., 2006, vol. 14, pp. 389–397.
Smirnova, T.A., Didenko, L.V., Andreev, A.L., Alekseeva, N.V., Stepanova, T.V., and Romanova, Yu.M., Electron microscopic study of Burkholderia cepacia biofilms, Microbiology (Moscow), 2008, vol. 77, no. 1, pp. 55–61.
Smirnova, T.A., Didenko, L.V., Azizbekyan, R.R., and Romanova, Yu.M., Structural and functional characteristics of bacterial biofilms, Microbiology (Moscow), 2010, vol. 79, no. 4, pp. 413–423.
Strelkova, E.A., Zhurina, M.V., Plakunov, V.K., and Belyaev, S.S., Stimulation of biofilm formation by antibiotics, Microbiology (Moscow), 2012, vol. 81, no. 2, pp. 259–262.
Strelkova, E.A., Pozdnyakova, N.V., Zhurina, M.V., Plakunov, V.K., and Belyaev, S.S., Role of the extracellular polymer matrix in resistance of bacterial biofilms to extreme environmental factors, Microbiology (Moscow), 2013, vol. 82, no. 2, pp. 119–125.
Su, Y., Zhang, X., Xia, F.-F., Zhang, Q.-Q., Kong, J.-Y., Wang, J., and He, R., Diversity and activity of methanotrophs in landfill cover soils with and without landfill gas recovery systems, Syst. Appl. Microbiol., 2014, vol. 37, pp. 200–207.
Tawakoli, P.N., Al-Ahmad, A., Hoth-Hannig, W., Hannig, M., and Hannig, C., Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm, Clin. Oral Invest., 2013, vol. 7, pp. 841–850.
Terada, A., Yamamoto, T., Tsuneda, S., and Hirata, A., Sequencing batch membrane bio?lm reactor for simultaneous nitrogen and phosphorus removal: novel application of membrane-aerated biofilm, Biotechnol. Bioeng., 2006, vol. 94, pp. 730–739.
Tojo, R., Suárez, A, Clemente, M.G., de los ReyesGavilán, C.G., Margolles, A., Gueimonde, M., and RuasMadiedo, P., Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis, World J. Gastroenterol., 2014, vol. 20, pp. 15163–15176.
Toté, K., Vanden Berghe, D., Maes, L., and Cos, P., A new colorimetric microtitre model for the detection of Staphylococcus aureus biofims, Lett. Appl. Microbiol., 2008, vol. 46, pp. 249–254.
Van Dam, A.A., Beveridge, M.C.M., Azim, M.E., and Verdegem, M.C.J., The potential of fish production based on periphyton, Rev. Fish. Biol. Fish., 2002, vol. 1, pp. 1–31.
Van den Driessche, F., Rigole, P., Brackman, G., and Coenye, T., Optimization of resazurin-based viability staining for quantification of microbial biofilms, J. Microbiol. Methods, 2014, vol. 98, pp. 31–34.
Vega, N.M. and Gore, J., Collective antibiotic resistance: mechanisms and implications, Curr. Opin. Microbiol., 2014, vol. 21, pp. 28–34.
Videla, H.A. and Herrera, L.K., Microbiologically influenced corrosion: looking to the future, Int. Microbiol., 2005, vol. 8, pp. 169–180.
Videla, H.A. and Herrera, L.K., Understanding microbial inhibition of corrosion. A comprehensive overview, Int. Biodeterior. Biodegr., 2009, vol. 63, pp. 896–900.
Vlaeminck, S.E., Terada, A., Smets, B.F., DeClippeleir, H., Schaubroeck, T., Bolca, S., Demeestere, L., Mast, J., Boon, N., Carballa, M., and Verstraete, W., Aggregate size and architecture determine microbial activity balance for one-stage partial nitritation and anammox, Appl. Environ. Microbiol., 2010, vol. 76, pp. 900–909.
Vornhagen, J., Stevens, M., McCormick, D., Dowd, S.E., Eisenberg, J.N.S., Boles, B.R., and Rickard, A.H., Coaggregation occurs amongst bacteria within and between domestic showerhead biofilms, Biofouling, 2013, vol. 29, pp. 53–68.
Walker, B., Kassim, K., and Stokes, L.D., The microbiome: a contributor to health and disease, J. Health Care Poor Underserved., 2015, vol. 26, pp. 62–72.
Weber, K., Delben, J., Bromage, T.G., and Duarte, S., Comparison of SEM and VPSEM imaging techniques with respect to Streptococcus mutans biofilm topography, FEMS Microbiol. Lett., 2014, vol. 350, pp. 175–179.
Weltzer, M.L. and Miller, S.R., Ecological divergence of a novel group of Chloroflexus strains along a geothermal gradient, Appl. Environ. Microbiol., 2013, vol. 79, pp. 1353–1358.
Williams, K.H., Bargar, J.R., Lloyd, J.R., and Lovley, D.R., Bioremediation of uranium-contaminated groundwater: a systems approach to subsurface biogeochemistry, Curr. Opin. Biotechnol.,2013, vol. 24, pp. 489–497.
Wolfaardt, G.M., Lawrence, J.R., Robarts, R.D., and Caldwell, D.E., Bioaccumulation of the herbicide diclofop in extracellular polymers and its utilization by a biofilm community during starvation, Appl. Environ. Microbiol., 1995, vol. 61, pp. 152–158.
Xie, Z., Thompson, A., Kashleva, H., and Dongari-Bagtzoglou, A., A quantitative real-time RT-PCR assay for mature C. albicans biofilms, BMC Microbiol., 2011, vol. 11, p. 93. http://wwwbiomedcentralcom/1471-2180/11/93
Xu, P. and Gunsolley, J., Application of metagenomics in understanding oral health and disease, Virulence, 2014, vol. 5, pp. 424–432.
Yang, L., Liu, Y., Wu, H., Høiby, N., Molin, S., and Song, Z., Current understanding of multi-species biofilms, Int. J. Oral. Sci., 2011, vol. 3, pp. 74–81.
Zarasvand, K.A. and Rai, V.R., Microorganisms: induction and inhibition of corrosion in metals, Int. Biodeterior. Biodegrad., 2014, vol. 87, pp. 66–74.
Zavarzin, G.A., Orleanskii, V.K., Gerasimenko, L.V., Pushko, S.N., and Ushatinskaya, G.T., Laboratory simulation of cyanobacterial mats of the alkaline geochemical barrier, Microbiology (Moscow), 2003, vol. 72, no. 1, pp. 80–85.
Zdorovenko, E.L., Shashkov, A.S., Zhurina, M.V., Plakunov, V.K., and Knirel, Y.A., Structure of the O-specific polysaccharides from planktonic and biofilm cultures of Pseudomonas chlororaphis 449, Carbohydr. Res., 2015, vol. 404, pp. 93–97.
Zhang, B., Halouska, S., Schiaffo, C.E., Sadykov, M.R., Somerville, G.A., and Powers, R., NMR analysis of a stress response metabolic signaling network, J. Proteom. Res., 2011, vol. 10, pp. 3743–3754.
Zhang, B. and Powers, R., Analysis of bacterial biofilms using NMR-based metabolomics, Future Med. Chem., 2012, vol. 4, pp. 1273–1306.
Zhurina, M.V., Kostrikina, N.A., Parshina, E.Yu., Strelkova, E.A., Yusipovich, A.I., Maksimov, G.V., and Plakunov, V.K., Visualization of the extracellular polymeric matrix of Chromobacterium violaceum biofilms by microscopic methods, Microbiology (Moscow), 2013, vol. 82, no. 4, pp. 517–524.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Nozhevnikova, E.A. Botchkova, V.K. Plakunov, 2015, published in Mikrobiologiya, 2015, Vol. 84, No. 6, pp. 623–644.
Rights and permissions
About this article
Cite this article
Nozhevnikova, A.N., Botchkova, E.A. & Plakunov, V.K. Multi-species biofilms in ecology, medicine, and biotechnology. Microbiology 84, 731–750 (2015). https://doi.org/10.1134/S0026261715060107
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261715060107