Abstract
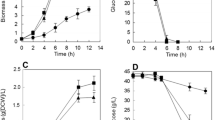
An expression plasmid was constructed in order to carry out heterologous expression of the gene of the NAD+-dependent formate dehydrogenase (FDH) from methylotrophic bacterium Moraxella sp. in the cells of Shewanella oneidensis MR-1 under aerobic and anaerobic conditions. In both modes of cell cultivation, recombinant FDH activity was revealed in the cell lysate of the transformants. In the medium with lać tate as a carbon source, the rate of anaerobic respiration determined as the rate of conversion of fumarate (the electron acceptor) to succinate was higher in the transformant with recombinant FDH. Anaerobic cultivation of the FDH-containing transformant of S. oneidensis MR-1 in a microbial fuel cell (MFC) revealed increased current density.
Similar content being viewed by others
References
Liu, C., Gorby, Y.A., Zachara, J.M., Fredrickson, J.K., and Brown, C.F., Reduction kinetics of Fe(III), Co(III), U(VI), Cr(VI), and Tc(VII) in cultures of dissimilatory metal-reducing bacteria, Biotechnol. Bioeng., 2002, vol. 80, no. 6, pp. 637–649.
Myers, C.R. and Myers, J.M., Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrifaciens MR-1, J. Bacteriol., 1992, vol. 174, pp. 3429–3438.
Debabov, V.G., Electricity from microorganisms, Microbiology, 2008, vol. 77, no. 2, pp. 123–131.
Heidelberg, J.F., Paulsen, I.T., Nelson, K.E., Gaidos, E.J., Nelson, W.C., Read, T.D., Eisen, J.A., Seshadri, R., Ward, N., Methe, B., Clayton, R.A., Meyer, T., Tsapin, A., Scott, J., Beanan, M., Brinkac, L., Daugherty, S., DeBoy, R.T., Dodson, R.J., Durkin, A.S., Haft, D.H., Kolonay, J.F., Madupu, R., Peterson, J.D., Umayam, L.A., White, O., Wolf, A.M., Vamathevan, J., Weidman, J., Impraim, M., Lee, K., Berry, K., Lee, C., Mueller, J., Khouri, H., Gill, J., Utterback, T.R., McDonald, L.A., Feldblyum, T.V., Smith, H.O., Venter, J.C., Nealson, K.H., and Fraser, C.M., Genome sequence of the dissimilatory metal ion-reducing bacterium shewanella oneidensis, Nature Biotechnol., 2002, vol. 20, pp. 1118–1123.
Shi, L., Lin, J.-T., Markillie, L.M., Squier, C., and Hooker, B.S., Overexpression of multi-heme C-type cytochromes, BioTechniques, 2005, vol. 38, no. 2, pp. 297–299.
Myers, C.R. and Myers, J.M., Replication of plasmids with the p15A origin in Shewanella putrefaciens MR-1, Lett. Appl. Microbiol., 1997, vol. 24, no. 3, pp. 221–225.
Mordkovich, N.N., Manuvera, V.A., Veiko, V.P., and Debabov, V.G., Uridine phosphorylase from Shewanella oneidensis MR-1: heterological expression, regulation, transcription, and properties, Appl. Biochem. Microbiol., 2012, vol. 48, no. 9, pp. 716–722.
Sambrook, J., Fritsch, E.F., and Maniatis, T., Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbor Laboratory Press, 1989.
Tang, Y., Meadows, A., Kirby, J., and Keasling, J., Anaerobic central metabolic pathways in Shewanella oneidensis MR-1 reinterpreted in the light of isotopic metabolite labeling, J. Bacteriol., 2007, vol. 189, no. 3, pp. 894–901.
Veiko, V.P., Ratmanova, K.I., Osipov, A.S., Bulenkov, M.T., and Pugachev, V.V., Directed introduction of amino groups at the internucleotide phosphate group for the solid-state synthesis of oligodeoxyribonucleotides, Bioorg. Khim., 1991, vol. 17, no. 5, pp. 685–689.
Shabalin, I.G., Filippova, E.V., Polyakov, K.M., Sadykhov, E.G., Safonova, T.N., Tikhonova, T.V., Tishkov, V.I., and Popov, V.O., Structures of the apo and holo forms of formate dehydrogenase from the bacterium Moraxella sp. C-1: towards understanding the mechanism of the closure of the interdomain cleft, Acta Crystallogr. D Biol. Crystallogr., 2009, vol. 65, no. 12, p. 1315.
Bradford, M.M., A rapid and sensitive method for the quantitation of microgram quantities protein utilizing the principle of protein dye binding, Anal. Biochem., 1976, vol. 2, pp. 246–254.
Il’in, V.K., Smirnov, I.A., Soldatov, P.E., Korshunov, D.V., Tyurin-Kuz’min, A.Yu., Starkova, L.V., Chumakov, P.E., Emel’yanova, L.K., Novikova, L.M., Debabov, V.G., and Voeikova, T.A., Microbial fuel cells as alternative eleć tric power sources, Kosm. Biol. Aviakosm. Med., 2012, vol. 46, no. 1, pp. 62–67.
Serres, M.H. and Riley, M., Genomic analysis of carbon source metabolism of Shewanella oneidensis MR-1: predictions versus experiments, J. Bacteriol., 2006, vol. 188, no. 13, pp. 4601–4609.
Pinchuk, G.E., Geydebrekht, O.V., Hill, E.A., Reed, J.L., Konopka, A.E., Beliaev, A.S., and Fredrickson, J.K., Pyruvate and lactate metabolism by Shewanella oneidensis MR-1 under fermentation, oxygen limitation and fumarate respiration conditions, Appl. Environ. Microbiol., 2011, vol. 77, no. 23, pp. 8234–8240.
Cordova, C.D., Schicklberger, M.F.R., Yu, Y., and Spormann, A.M., Partial functional replacement of CymA by SirCD in Shewanella oneidensis MR-1, J. Bacteriol., 2011, vol. 193, no. 9, pp. 2312–2321.
McMillan, D.G.G., Maritt, S.J., Butt, J.N., and Jeuken, L.J.C., Menaquinone-7 is specific cofactor in tetraheme quinol dehydrogenase CymA, J. Biol. Chem., 2012, vol. 287, no. 17, pp. 14215–14225.
Berrios-Rivera, S.J., Bennett, G.N., and San Ka-Yui, Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD+-dependent formate dehydrogenase, Metabolic Engineering, 2002, vol. 4, pp. 217–229.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.N. Mordkovich, T.A. Voeikova, L.M. Novikova, I.A. Smirnov, V.K. Il’in, P.E. Soldatov, A.Yu. Tyurin-Kuz’min, T.S. Smolenskaya, V.P. Veiko, R.S. Shakulov, V.G. Debabov, 2013, published in Mikrobiologiya, 2013, Vol. 82, No. 4, pp. 395–401.
Rights and permissions
About this article
Cite this article
Mordkovich, N.N., Voeikova, T.A., Novikova, L.M. et al. Effect of NAD+-dependent formate dehydrogenase on anaerobic respiration of Shewanella oneidensis MR-1. Microbiology 82, 404–409 (2013). https://doi.org/10.1134/S0026261713040061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261713040061