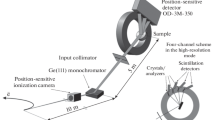
Abstract
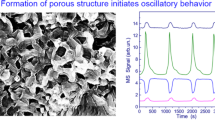
The catalytic oxidation of methane over a nickel foil has been investigated in a wide range of methane: oxygen molar ratios at a constant reactor temperature of 680°C. Under oxygen-deficient conditions, the reaction takes place in the self-oscillating mode. Stable self-sustained oscillations of the methane oxidation rate are observed in the CH4: O2 molar ratio range from 2: 1 to 19: 1. This process is accompanied by catalyst temperature oscillations, whose amplitude reaches 80°C. It has been demonstrated by scanning electron microscopy that the origination of the self-sustained oscillations is accompanied by morphological changes in the catalyst surface. Under the action of the reaction medium, a porous layer 5–10 µm in thickness forms on the nickel foil surface. The mechanism of methane oxidation over nickel that accounts for the onset of the self-sustained oscillations is discussed. This mechanism is based on periodical nickel oxidation and reduction. When nickel is in the high-activity state, it is mainly in metallic form, and the passage of nickel into its low-activity state is accompanied by the formation of a nickel oxide layer on the foil surface. The reduction of this nickel oxide causes a periodic decrease in the catalyst temperature. The total and partial oxidations of methane on the reduced surface of the nickel foil raise the catalyst temperature. Under oxygen-deficient conditions, a carbon deposit builds up on the catalyst surface, and the combustion of this deposit complicates the catalyst temperature oscillation profile.
Similar content being viewed by others
References
Schüth, F., Henry, B.E., and Schmidt, L.D., Adv. Catal., 1993, vol. 39, p. 51.
Slinko, M.M. and Jaeger, N.I., Stud. Surf. Sci. Catal., 1994, vol. 86, p. 1.
Imbihl, R. and Ertl, G., Chem. Rev., 1995, vol. 95, p. 697.
Epstein, I.R. and Showalter, K., J. Phys. Chem., 1996, vol. 100, p. 13132.
Zhang, X., Mingos, D.M.P., and Hayward, D.O., Catal. Lett., 2001, vol. 72, p. 147.
Tulenin, Y.P., Sinev, M.Y., Savkin, V.V., and Korchak, V.N., Catal. Today, 2004, vol. 91–92, p. 155.
Zhang, X., Hayward, D.O., and Mingos, D.M.P., Catal. Lett., 2002, vol. 83, p. 149.
Zhang, X., Hayward, D.O., and Mingos, D.M.P., Catal. Lett., 2003, vol. 86, p. 235.
Zhang, X., Lee, C.S.M., Mingos, D.M.P., and Hayward, D.O., Appl. Catal. A, 2003, vol. 248, p. 129.
Liu, Y., Fang, W.P., Weng, W.Z., and Wan, H.L., J. Mol. Catal. A: Chem., 2005, vol. 239, p. 193.
Li, J.-M., Huang, F.-Y., Weng, W.-Z., Pei, X.-Q., Luo, C.-R., Lin, H.-Q., Huang, C.-J., and Wan, H.-L., Catal. Today, 2008, vol. 131, p. 179.
Zhang, X., Lee, C.S.M., Mingos, D.M.P., and Hayward, D.O., Appl. Catal. A, 2003, vol. 240, p. 183.
Wang, M., Weng, W., Zheng, H., Yi, X., Huang, C., and Wan, H., J. Nat. Gas Chem., 2009, vol. 18, p. 300.
Kaichev, V.V., Sorokin, A.M., Timoshin, A.I., and Vovk, E.I., Instrum. Exp. Tech., 2002, vol. 45, no. 1, p. 50.
Khromova, S.A., Smirnov, A.A., Bulavchenko, O.A., Saraev, A.A., Kaichev, V.V., Reshetnikov, S.I., and Yakovlev, V.A., Appl. Catal., A, 2014, vol. 470, p. 261.
Nilsen, O., Kjekshus, A., and Fjellvåg, H., Appl. Catal. A, 2001, vol. 207, p. 43.
Hannevold, L., Nilsen, O., Kjekshus, A., and Fjellvåg, H., Appl. Catal. A, 2005, vol. 284, p. 163.
Miller, A.V., Kaichev, V.V., Prosvirin, I.P., and Bukhtiyarov, V.I., J. Phys. Chem. C, 2013, vol. 117, p. 8189.
Kaichev, V.V., Prosvirin, I.P., and Bukhtiyarov, V.I., Kinet. Catal., 2014, vol. 55, no. 4, p. 509.
Matveev, A.V., Kaichev, V.V., Saraev, A.A., Gorodetskii, V.V., Knop-Gericke, A., Bukhtiyarov, V.I., and Nieuwenhuys, B.E., Catal. Today, 2015, vol. 244, p. 29.
Lashina, E.A., Kaichev, V.V., Chumakova, N.A., Ustyugov, V.V., Chumakov, G.A., and Bukhtiyarov, V.I., Kinet. Catal., 2012, vol. 53, no. 3, p. 374.
Hickman, D.A. and Schmidt, L.D., Science, 1993, vol. 259, p. 343.
Bychkov, V.Y., Tyulenin, Y.P., Korchak, V.N., and Aptekar, E.L., Appl. Catal. A, 2006, vol. 304, p. 21.
McKay, J.M. and Henrich, V.E., Phys. Rev. B: Condens. Matter, 1985, vol. 32, p. 6764.
Dietz, R.E., Parisot, G.I., and Meixner, A.E., Phys. Rev. B: Condens. Matter, 1971, vol. 4, p. 2302.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Saraev, S.S. Kosolobov, V.V. Kaichev, V.I. Bukhtiyarov, 2015, published in Kinetika i Kataliz, 2015, Vol. 56, No. 5, pp. 606–613.
Rights and permissions
About this article
Cite this article
Saraev, A.A., Kosolobov, S.S., Kaichev, V.V. et al. Origin of temperature oscillations of nickel catalyst occurring in methane oxidation. Kinet Catal 56, 598–604 (2015). https://doi.org/10.1134/S002315841505016X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315841505016X