Abstract
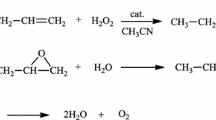
The homogeneous oxidation of 1,3-butadiene (BD) in H2O2-HPC-CH3CN (HPC = heteropoly compound) solutions has been investigated. The route of the reaction depends on the nature of the metal capable of coordinating with active oxygen in the HPC. The products of radical BD oxidation (acrolein, 3-butene-1,2-diol, 2-butene-1,4-diol, furan) form in the presence of H3+n PMo12 − n V n O40 (n = 1, 2) acids. 3,4-Epoxy-1-butene (EB) and acrolein + furan, which form in equal amounts in the presence of the (n-Bu4N)5PW11O39Fe(OH) salt, result, respectively, from the electrophilic addition of hydrogen peroxide to BD and from radical BD oxidation on iron-oxygen complexes in the HPC composition. The reaction carried out in the presence of (n-Bu4N)3{PO4[WO(O2)2]4}, (n-Bu4N)5Na0.6H1.4PW11O39, or (EMIm)5NaHPW11O39 yields EB with high selectivity on the reacted BD basis (up to 97%) and H2O2 (about 100%). The formation and conversion of the phosphotungstate peroxo complexes PW n O α− m (n = 2, 3, 4) that are active in BD epoxidation have been investigated by 31PNMR spectroscopy. The role of the tetrabutylammonium and ethylmethylimidazolium cations in the formation of these complexes has been demonstrated.
Similar content being viewed by others
References
Monnier, J.R., Appl. Catal., A, 2001, vol. 221, nos. 1–2, p. 73.
Blanko-Brieva, G., Capel-Sanchez, M.C., Pilar de Frutos, M., Padilla-Polo, A., Campos-Martin, J.M., and Fierro, J.L.G., Ind. Eng. Chem. Res., 2008, vol. 47, no. 21, p. 8011.
Mizuno, N., Yamaguchi, K., and Kamata, K., Coord. Chem. Rev., 2005, vol. 249, nos. 17–18, p. 1944.
Mizuno, N. and Kamata, K., Coord. Chem. Rev., 2011, vol. 255, nos. 19–20, p. 2358.
Mirkhani, V., Moghadam, M., Tangestaninejad, S., Mohammodpoor-Baltork, I., Shams, E., and Rosouli, N., Appl. Catal., A, 2008, vol. 334, no. 1, p. 106.
Zhao, W., Ma, B., Hua, H., Zhang, Y., and Ding, Y., Catal. Commun., 2008, vol. 9, no. 14, p. 2455.
Gharah, N., Chowdhury, K., Mukherjee, M., and Bhattacharyya, R., Trans. Met. Chem., 2008, vol. 33, no. 5, p. 635.
Liu, L., Chen, C., Hu, X., Mohamood, T., Ma, W., Lin, J., and Zhao, J., New J. Chem., 2008, vol. 32, no. 2, p. 283.
Zhang, Z., Zhao, W., Ma, B., and Ding, Y., Catal. Commun., 2010, vol. 12, no. 4, p. 318.
Zhao, W., Ding, Y., Zhang, Z., Ma, B., and Qiu, W., React. Kinet. Mech. Catal., 2011, vol. 102, no. 1, p. 93.
Li, H., Yunxiang, Q., Yinyin, Y., Wenwen, Z., Ting, C., Yu, S., Huan, L., Bo, F., and Zhenshan, H., New J. Chem., 2011, vol. 35, no. 9, p. 1836.
Yan, L., Jun, W., Dunru, Z., Mingjue, Z., Pingping, Z., Zhouyang, L., and Jun, H., Green Chem., 2011, vol. 13, no. 7, p. 1636.
Antonova, N.S., Carbo, J.J., Kortz, U., Kholdeeva, O.A., and Poblet, J.M., J. Am. Chem. Soc., 2010, vol. 132, no. 21, p. 7488.
Gharnati, L., Doring, M., and Arnold, U., Curr. Org. Synth., 2009, vol. 6, no. 4, p. 342.
Kuznetsova, N.I., Detusheva, L.G., Kuznetsova, L.I., Fedotov, M.A., and Likholobov, V.A., Kinet. Catal., 1992, vol. 33, no. 3, p. 415.
Mimoun, H., Saussine, L., Daire, E., Postel, M., Fisher, J., and Weiss, R., J. Am. Chem. Soc., 1983, vol. 105, no. 10, p. 3101.
Dhakshinamoorthy, A. and Pitchumani, K., Tetrahedron: Asymmetry, 2006, vol. 62, no. 42, p. 9911.
Saedi, Z., Tangestaninejad, S., Moghadam, M., Mirkhani, V., and Mohammadpoor, I., Catal. Commun., 2012, vol. 17, no. 1, p. 18.
Adam, W., Corma, A., Martinez, A., and Renz, M., Chem. Ber., 1996, vol. 129, no. 12, p. 1453.
Brooks, C.D., Huang, L., McCarron, M., and Johnstone, A.W., Chem. Commun., 1999, no. 1, p. 37.
Centi, G. and Trifiro, F., J. Mol. Catal., 1986, vol. 35, no. 2, p. 255.
Trifiro, F. and Jiru, P., Catal. Today, 1988, vol. 3, no. 3, p. 519.
Schroeder, W.D., Fontenot, C.J., and Schrader, G.L., J. Catal., 2001, vol. 203, no. 2, p. 382.
Takehira, K., Mimoun, H., and De Roch, I.S., J. Catal., 1979, vol. 58, no. 2, p. 155.
Hotanahalli, S.S. and Chandalia, S.B., J. Appl. Chem., 1970, vol. 20, p. 323.
US Patent 4172838, 1979.
US Patent 4243593, 1981.
US Patent 4298531, 1981.
Zang, X., Zang, Z., Suo, J., and Li, Sh., Catal. Lett., 2000, vol. 66, no. 3, p. 175.
Kamata, K., Katani, M., Yamaguchi, K., Hikichi, H., and Mizuno, N., Chem. Eur. J., 2007, vol. 13, no. 2, p. 639.
Neumann, R. and de la Vega, M., J. Mol. Catal., 1993, vol. 84, no. 1, p. 93.
Nomiya, K., Yanagibayashi, H., Nozaki, C., Kondoh, K., Hiramatsu, E., and Shimizu, Y., J. Mol. Catal. A: Chem., 1996, vol. 114, nos 1–3, p. 181.
Kuznetsova, L.I., Maksimovskaya, R.I., and Fedotov, M.A., Izv. Akad. Nauk SSSR, Ser. Khim., 1985, no. 2, p. 488.
Venturello, C., D’Aloisio, R., Bart, J.C.J., and Ricci, M., J. Mol. Catal., 1985, vol. 32, no. 1, p. 107.
Ishii, Y., Jamawaki, K., Yoshida, T., Ura, T., and Ogawa, M., J. Org. Chem., 1987, vol. 52, p. 1868.
Aubry, C., Chottard, G., Platzer, N., Bregeault, J.-M., Thouvenot, R., Chauveau, F., Huet, C., and Ledon, H., Inorg. Chem., 1991, vol. 30, no. 23, p. 4409.
Salles, L., Aubry, C., Thouvenot, R., Robert, F., Doremieux-Morin, C., Chottard, G., Ledon, H., Jeannin, Y., and Bregeault, J.-M., Inorg. Chem., 1994, vol. 33, no. 5, p. 871.
Duncan, D.C., Chambers, R.C., Hecht, E., and Hill, C.L., J. Am. Chem. Soc., 1995, vol. 117, no. 2, p. 681.
Dengel, A.C., Griffith, W.P., and Parkin, B.C., J. Chem. Soc., Dalton Trans., 1993, no. 18, p. 2683.
Polotebnova, N.A., Nguen Van Cheu, and Kal’nibolotskaya, V.V., Zh. Neorg. Khim., 1973, vol. 18, no. 2, p. 413.
Maksimov, G.M., Kustova, G.N., Matveev, K.I., and Lazarenko, T.P., Koord. Khim., 1989, vol. 15, no. 6, p. 788.
Kuznetsova, L.I., Kuznetsova, N.I., Maksimovskaya, R.I., Aleshina, G.I., Koscheeva, O.S., and Utkin, V.A., Catal. Lett., 2011, vol. 141, no. 10, p. 1442.
Kozhevnikov, I.V., Catalysis for Fine Chemical Synthesis, vol. 2: Catalysis by Polyoxometalates, Chichester: Wiley, 2002.
Hendry, D.G., Mayo, R.F., and Scheutzle, D., Ind. Eng. Chem. Res., 1968, vol. 7, no. 2, p. 136.
Odyakov, V.F., Kuznetsova, L.I., and Matveev, K.I., Zh. Neorg. Khim., 1978, vol. 23, no. 2, p. 457.
Mel’nik, L.V., Srednev, S.S., Rybina, G.V., Meshechkina, F.E., Shevchuk, F.S., and Danilova, A.S., Pet. Chem., 2007, vol. 47, no. 3, p. 201.
Kuznetsova, L.I., Detusheva, L.G., Fedotov, M.A., and Likholobov, V.A., J. Mol. Catal. A: Chem., 1996, vol. 111, nos. 1–2, p. 81.
Kuznetsova, N.I., Kuznetsova, L.I., and Likholobov, V.A., J. Mol. Catal. A: Chem., 1996, vol. 108, nos. 1–3, p. 135.
Behar, D., Gonzalez, C., and Neta, P., J. Phys. Chem. A, 2001, vol. 105, no. 32, p. 7607.
Wang, A. and Jiang, H., J. Org. Chem., 2010, vol. 75, no. 7, p. 2321.
Metelitsa, D.I., Modelirovanie okislitel’no-vosstanovitel’nykh fermentov (Modeling of Redox Enzymes), Minsk: Nauka i Tekhnika, 1984.
Kozlov, Yu.N., Nizova, G.V., and Shul’pin, G.B., Russ. J. Phys. Chem., 2004, vol. 78, no. 2, p. 184.
Kozlov, Yu.N., Nizova, G.V., and Shul’pin, G.B., J. Mol. Catal. A: Chem., 2005, vol. 227, nos. 1–2, p. 247.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.I. Kuznetsova, N.I. Kuznetsova, R.I. Maksimovskaya, O.S. Koshcheeva, V.A. Utkin, 2013, published in Kinetika i Kataliz, 2013, Vol. 54, No. 4, pp. 442–452.
Rights and permissions
About this article
Cite this article
Kuznetsova, L.I., Kuznetsova, N.I., Maksimovskaya, R.I. et al. Catalytic properties of heteropoly compounds in 1,3-butadiene oxidation with hydrogen peroxide. Kinet Catal 54, 420–430 (2013). https://doi.org/10.1134/S0023158413040071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158413040071