Abstract
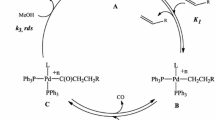
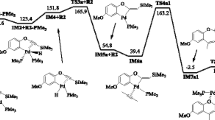
Cyclohexene hydrocarbomethoxylation catalyzed by Pd(OAc)2-p-toluenesulfonic acid-diphosphine systems has been investigated for a wide range of diphosphine structures and concentrations. The factors controlling the activity of the palladium-containing catalysts include the hydrocarbon moiety of the ligand and the mutual arrangement of the phosphine groups. A comparison between the promoting effects of monophosphine and diphosphine ligands has demonstrated that bridged trans-diphosphines are more efficient in kinetic and concentration terms (TOF and P/Pd ratio, respectively). In particular, the promoting activity of diphosphines is one order of magnitude higher than that of triphenylphosphine, and this effect is attained at 8–65 times lower P/Pd ratios. It is discussed how the catalytic properties of the systems depend on the chelate effect and on the geometric compatibility between the diphosphine structure and the arrangement of vacant s and d orbitals of the palladium center.
Similar content being viewed by others
References
Nozaki, K. and Ojima, I., Catalytic Asymmetric Synthesis, New York: Wiley, 2000.
Drent, E. and Buzelaar, P.H.M., Chem. Rev., 1996, vol. 96, p. 663.
Clegy, W., Eastham, G.R., Elsegood, M.R., Tooze, R.P., Wang, X.L., and Whiston, K., Chem. Commun., 1999, p. 1877.
Kron, T.E., Terekhova, M.I., Noskov, Yu.G., and Petrov, E.S., Kinet. Catal., 2001, vol. 42, no. 2, p. 182.
Aver’yanov, V.A., Batashev, S.A., Sevost’yanova, N.T., and Nosova, N.M., Kinet. Catal., 2006, vol. 47, no. 3, p. 375.
Kiss, G., Chem. Rev., 2001, vol. 101, no. 11, p. 3435.
Aver’yanov, V.A., Nosova, N.M., and Batashev, S.A., Pet. Chem., 2006, vol. 46, no. 2, p. 99.
Del Rio, I., Ruiz, N., Claver, C., van der Veen, L., and van Leenwen, P.W.N.M., J. Mol. Catal. A: Chem., 2000, vol. 161, p. 39.
Fanjul, T., Eeastham, G., Fey, N., Hamilton, A., Orpen, A.G., Pringle, P.G., and Waugh, M., Organometallics, 2010, vol. 29, p. 2292.
Guiu, E., Caporali, M., Munoz, D., Muller, C., Lutz, M., Spek, A.L., Claver, C., and van Leeuwen, P.W.N.M., Organometallics, 2006, vol. 25, p. 3102.
Van Leenwen, P.W.N.M., Kamer, P.C.J., Reek, J.N.H., and Dierkes, P., Chem. Rev., 2000, vol. 100, p. 2741.
Bricklebank, N., Godfrey, S.M., and McAuliffe, C.A., J. Chem. Soc., Dalton Trans., 1998, p. 2379.
Hayashi, T., Tanaka, M., Ikeda, Y., and Ogata, I., Bull. Chem. Soc. Jpn., 1979, vol. 52, no. 9, p. 2605.
Hayashi, T., Kawabata, Y., Isoyama, T., and Ogata, I., Bull. Chem. Soc. Jpn., 1981, vol. 54, no. 11, p. 3438.
Nifant’ev, I.E., Bagrov, V.V., Batashev, S.A., Sevostyanova, N.T., Vorobiev, A.A., Averyanov, V.A., Toloraya, S.A., and Tavtorkin, A.N., J. Mol. Catal. A: Chem., 2011, vol. 350, nos. 1–2, p. 64.
Doebler, Chr. and Kreuzfeld, H.-J., J. Prakt. Chem., 1981, vol. 323, no. 4, p. 667.
Dang, T.P., Poulin, J.-C., and Kagan, H.B., J. Organomet. Chem., 1975, vol. 91, p. 105.
Doherty, S., Robins, E.G., Knight, J.G., Newman, C.R., Rhodes, B., Champkin, P.A., and Clegg, W., J. Organomet. Chem., 2001, vol. 640, p. 182.
Tavtorkin, A.N., Sofia, A., Toloraya, S.A., Nifant’ev, E.E., and Nifant’ev, I.E., Tetrahedron Lett., 2011, vol. 52, p. 824.
Clark, P.W. and Mulraney, B.J., J. Organomet. Chem., 1981, vol. 217, no. 1, p. 51.
US Patent 4960906, 1989.
El’man, A.R., Matveev, V.A., Slivinskii, E.V., and Loktev, S.M., Pharm. Chem. J., 1990, vol. 24, no. 3, p. 217.
Gusev, O.V., Kalsin, A.M., Peterleither, M.G., Petrovskii, P.P., and Lissenko, K.A., Organometallics, 2002, vol. 21, p. 3637.
Kiselev, Yu.M. and Dobrynina, N.A., Khimiya koordinatsionnykh soedinenii (Chemistry of Coordination Compounds), Moscow: Akademiya, 2007.
Skopenko, V.V., Tsivadze, A.Yu., Savranskii, L.I., and Garnovskii, A.D., Koordinatsionnaya khimiya (Coordination Chemistry), Moscow: Akademkniga, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.E. Nifant’ev, S.A. Batashev, S.A. Toloraya, A.N. Tavtorkin, N.T. Sevost’yanova, A.A. Vorob’ev, V.V. Bagrov, V.A. Aver’yanov, 2012, published in Kinetika i Kataliz, 2012, Vol. 53, No. 4, pp. 483–490.
Rights and permissions
About this article
Cite this article
Nifant’ev, I.E., Batashev, S.A., Toloraya, S.A. et al. Steric and electronic factors in the promoting activity of diphosphine ligands in cyclohexene hydrocarbomethoxylation catalyzed by palladium acetate. Kinet Catal 53, 462–469 (2012). https://doi.org/10.1134/S0023158412040076
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158412040076