Abstract
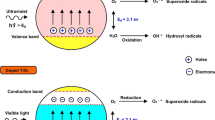
The results of many-year studies of the relationship between the physical properties and photocatalytic activity of TiO2 and Pt/TiO2 in photocatalytic purification and disinfection of air and water and water photodecomposition with oxygen evolution are presented. Recommendations are given as to finding the optimal method for platinum supporting on TiO2 to achieve the highest possible catalytic activity. Multisite kinetic models of the gas-phase oxidation of simple organic substances are considered. Methods for regenerating the photocatalyst after its deactivation in the oxidation of sulfur-containing organic substances are suggested. New data are discussed on the acceleration of air purification by the combination of photocatalytic oxidation with atmospheric electric discharges, the addition of gaseous hydrogen peroxide, and oxidation on photocatalysts existing in the aerosol state. As compared to pure TiO2, platinated titanium dioxide has a higher capability for disinfection and complete mineralization of microorganisms. Two promising methods for production of hydrogen from water using solar light are presented.
Similar content being viewed by others
References
Parmon, V.N., in Fotokataliticheskoe preobrazovanie solnechnoi energii: Geterogennye, gomogennye i molekulyarnye strukturno-organizovannye sistemy (Photocatalytic Conversion of Solar Energy: Heterogeneous, Homogeneous, and Molecular Structured Systems), Novosibirsk: Nauka, 1991, p. 7.
Vorontsov, A.V., Usp. Khim., 2008, vol. 77, p. 973.
Nikazar, M., Golivand, K., and Makhanpur, K., Kinet. Katal., 2007, vol. 48, p. 230 [Kinet. Catal. (Engl. Transl.), vol. 48, p. 214].
Shi, Dzh., Zheng, Dzh., Khu, Ya., and Zhao, Yu., Kinet. Katal., 2008, vol. 49, p. 293 [Kinet. Catal. (Engl. Transl.), vol. 49, p. 279].
Jothiramalingam, R., Tsao, T.M., and Wang, M.K., Kinet. Katal., 2009, vol. 50, p. 771 [Kinet. Catal. (Engl. Transl.), vol. 50, p. 741].
Zenkovets, G.A., Gavrilov, V.Yu., Shutilov, A.A., and Tsybulya, S.V., Kinet. Katal., 2009, vol. 50, p. 790 [Kinet. Catal. (Engl. Transl.), vol. 50, p. 760].
Aikin Chzhan, Nin Chzhan, Sanguo Khon, and Min Chzhan, Kinet. Katal., 2009, vol. 50, p. 778 [Kinet. Catal. (Engl. Transl.), vol. 50, p. 748].
Renz, C., Helv. Chim. Acta, 1921, vol. 4, p. 961.
Fujishima, A., Hashimoto, K., and Watanabe, T., TiO 2 Photocatalysis, Tokyo: Bkc, 1999.
Ireland, J.C., Klostermann, P., Rice, E.W., and Clark, R.M., Appl. Environ. Microbiol., 1993, vol. 59, p. 1668.
Jacoby, W.A., Maness, P.C., Wolfrum, E.J., Blake, D.M., and Fennell, J.A., Environ. Sci. Technol., 1998, vol. 32, p. 2650.
Fujishima, A. and Honda, K., Nature, 1972, vol. 238, p. 37.
Vorontsov, A.V., Altynnikov, A.A., Savinov, E.N., and Kurkin, E.N., J. Photochem. Photobiol. A, 2001, vol. 144, p. 193.
Vorontsov, A.V., Savinov, E.N., Barannik, G.B., Troitsky, V.N., and Parmon, V.N., Catal. Today, 1997, vol. 39, p. 207.
Vorontsov, A.V. and Dubovitskaya, V.P., J. Catal., 2004, vol. 221, p. 102.
Bedilo, A.F. and Volodin, A.M., Kinet. Katal., 2009, vol. 50, p. 332 [Kinet. Catal. (Engl. Transl.), vol. 50, p. 314].
Vorontsov, A.V., Catal. Commun., 2007, vol. 8, p. 2100.
Parmon, V.N and Zamaraev, K.I, in Handbook of Heterogeneous Catalysis, Ertl, G., Knozinger, H., and Weitkamp, J., Eds., 1997, vol. 4, p. 686.
Besov, A.S., Vorontsov, A.V., and Parmon, V.N., Appl. Catal., B, 2009, vol. 89, p. 602.
Besov, A.S., Trubitsyn, D.A., and Vorontsov, A.V., Katal. Prom-sti., 2010, no. 1, p. 35.
Watts, R.J., Kong, S., Orr, M.P., Miller, G.C., and Henry, B.E., Water Res., 1995, vol. 29, p. 95.
Sichel, C., Cara, M., Tello, J., Blanco, J., and Fernández-Iáez, P., Appl. Catal., B, 2007, vol. 74, p. 152.
Jacoby, W.A., Maness, P.C., Wolfrum, E.J., Blake, D.M., and Fennell, J.A., Environ. Sci. Technol., 1998, vol. 32, p. 2650.
Pal, A., Pehkonen, S.O., Yu, L.E., and Ray, M.B., J. Photochem. Photobiol., A, 2007, vol. 186, p. 335.
Kozlova, E.A., Korobkina, T.P., Vorontsov, A.V., and Parmon, V.N., Appl. Catal., A, 2009, vol. 367, p. 130.
Kozlova, E.A., Safatov, A.S., Kiselev, S.A., Marchenko, V.Yu., Sergeev, A.A., Skarnovich, M.O., Emelyanova, E.K., Smetannikova, M.A., Buryak, G.A., and Vorontsov, A.V., Environ. Sci. Technol., 2010, vol. 41, p. 5121.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.V. Vorontsov, E.A. Kozlova, A.S. Besov, D.V. Kozlov, S.A. Kiselev, A.S. Safatov, 2010, published in Kinetika i Kataliz, 2010, Vol. 51, No. 6, pp. 829–836.
Rights and permissions
About this article
Cite this article
Vorontsov, A.V., Kozlova, E.A., Besov, A.S. et al. Photocatalysis: Light energy conversion for the oxidation, disinfection, and decomposition of water. Kinet Catal 51, 801–808 (2010). https://doi.org/10.1134/S0023158410060042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158410060042