Abstract
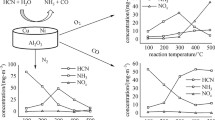
Selective CO oxidation in a mixture simulating the methanol steam reforming product with an air admixture was studied over Ru/Al2O3 catalysts in a quasi-adiabatic reactor. On-line monitoring of the gas temperature in the catalyst bed and of the residual CO concentration at different reaction conditions made it possible to observe the ignition and quenching of the catalyst surface, including transitional regimes. A sharp decrease in the residual CO concentration takes place when the reaction passes to the ignition regime. The evolution of the temperature distribution in the catalyst bed in the ignition regime and the specific features of the steady-state and transitional regimes are considered, including the effect of the sample history. In selective CO oxidation and in H2 oxidation in the absence of CO, the catalyst is deactivated slowly because of ruthenium oxidation. In both reactions, the deactivated catalyst can be reactivated by short-term treatment with hydrogen. A 0.1% Ru/Al2O3 catalyst is suggested. In the surface ignition regime, this catalyst can reduce the residual CO concentration from 0.8 vol % to 10–15 ppm at O2/CO = 1 even in the presence of H2O and CO2 (up to ∼20 vol %) at a volumetric flow rate of ∼100 1 (g Cat)−1 h−1, which is one magnitude higher than the flow rates reported for this process in the literature.
Similar content being viewed by others
References
Edwards, N., Ellis, S.R., Frost, J.C., Golunski, S.E., van Keulen, A.N.J., Lindewald, N.G., and Reinkingh, J.G., J. Power Sources, 1998, vol. 71, p. 123.
US Patent 6576208, 2003.
Echigo, M. and Tabata, T., Appl. Catal., A, 2003, vol. 251, p. 157.
Snytnikov, P.V., Extended Abstract of Cand. Sci. (Chem.) Dissertation, Novosibirsk: Inst. of Catalysis, 2004.
Eur. Patent 1 485 202, 2003.
Snytnikov, P.V., Sobyanin, V.A., Belyaev, V.D., Tsyrulnikov, P.G., Shitova, N.B., and Shlyapin, D.A., Appl. Catal., A, 2003, vol. 239, p. 149.
Kawatsu, S., J. Power Sources, 1998, vol. 71, p. 150.
Dudfield, C.D., Chen, R., and Adcock, P.L., J. Power Sources, 2000, vol. 86, p. 214.
Kipnis, M.A., Volnina, E.A., Samokhin, P.V., Lin, G.I., and Rozovskii, A.Ya., Tezisy dokl. 1 Vseross. konf. “Khimiya dlya avtomobil’nogo transporta” (Proc. 1st Russian Conf. on Chemistry for Automotive Applications), Novosibirsk, 2004, pp. 199.
Dudfield, C.D., Chen, R., and Adcock, P.L., J. Power Sources, 2000, vol. 85, p. 237.
Rosso, I., Galletti, C., Saracco, G., Garrone, E., and Specchia, V., Appl. Catal., B, 2004, vol. 48, p. 195.
Worner, A., Friedrich, C., and Tamme, R., Appl. Catal., A, 2003, vol. 245, p. 1.
Han, Y.-F., Kahlich, M.J., Kinne, M., and Behm, R.J., Phys. Chem. Chem. Phys., 2002, vol. 4, p. 389.
Rozovskii, A. Ya., Kipnis, M.A., Volnina, E.A., Lin, G.I., and Samokhin, P.V., Kinet. Katal., 2004, vol. 45, no. 4, p. 654 [Kinet. Catal. (Engl. Transl.), vol. 45, no. 4, p. 618].
Rozovskii, A. Ya., Kipnis, M.A., Volnina, E.A., Lin, G.I., and Samokhin, P.V., Kinet. Katal. (in press).
Frank-Kamenetskii, D.A., Diffuziya i teploperedacha v khimicheskoi kinetike (Diffusion and Heat Transfer in Chemical Kinetics), Moscow: Nauka, 1967.
Rozovskii, A.Ya., Kinetika topokhimicheskikh reaktsii (Kinetics of Topochemical Reactions), Moscow: Nauka, 1980.
Zhilyaeva, N.A., Volnina, E.A., Shuikina, L.P., and Frolov, V.M., Neftekhimiya, 2000, vol. 40, no. 6, p. 422 [Pet. Chem. (Engl. Transl.), vol. 40, no. 6, p. 383].
Kiss, J.T. and Gonzalez, R.D., J. Phys. Chem., 1984, vol. 88, p. 892.
Amann, J., Crihan, D., Knapp, M., Lundgren, E., Loffler, E., Muhler, M., Narknede, V., Over, H., Schmid, M., Seitsonen, A.P., and Varga, P., Angew. Chem., Int. Ed. Engl., 2005, vol. 44, p. 917.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.Ya. Rozovskii, M.A. Kipnis, E.A. Volnina, P.V. Samokhin, G.I. Lin, 2008, published in Kinetika i Kataliz, 2008, Vol. 49, No. 1, pp. 99–109.
The article includes materials from the authors’ report at the II Russian Conference on Current Problems in Petroleum Chemistry, Ufa, October 11–13, 2005.
Rights and permissions
About this article
Cite this article
Rozovskii, A.Y., Kipnis, M.A., Volnina, E.A. et al. Selective CO oxidation on a Ru/Al2O3 catalyst in the surface ignition regime: 1. Fine purification of hydrogen-containing gases. Kinet Catal 49, 92–102 (2008). https://doi.org/10.1134/S0023158408010114
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158408010114