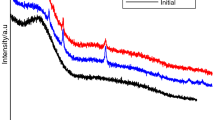
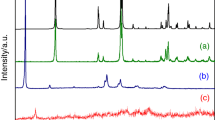
Abstract
We have studied the hydrolytic stability of titanyl hydrogen phosphates in relation to their composition, the synthesis procedure, and the nature and concentration of the electrolyte. The chemical stability of these compounds can be improved by increasing the electrolyte concentration and decreasing the degree of hydration of metal cations in solution and also by modifying their composition with metal cations differing in acid–base properties from titanium(IV). We have found conditions that maximize the hydrolytic stability of the titanyl hydrogen phosphates.
Similar content being viewed by others
References
Elghniji, K., Saad, M.E.K., Araissi, M., Elaloui, E., and Moussaoui, Y., Chemical modification of TiO2 by anions using the sol–gel route with controlled precipitation and hydrolysis: enhancing thermal stability, Mater. Sci.–Pol., 2014, vol. 32, no. 4, pp. 617–625.
Ortiz-Oliveros, H.B., Flores-Espinosa, R.M., Ordonez-Regil, E., and Fernandez-Valverde, S.M., Synthesis of α-Ti(HPO4)2 · H2O and sorption of Eu(III), Chem. Eng. J., 2014, vol. 236, pp. 398–405.
García-Glez, J., Trobajo, C., Khainakov, S.A., and Amghouz, Z., α-Titanium phosphate intercalated with propylamine: an alternative pathway for efficient europium( III) uptake into layered tetravalent metal phosphates, Arab. J. Chem., 2017, no. 10, pp. 885–894.
Baig, U., Khan Rao, R.A., Khan, A.A., Sanagi, M.M., and Gondal, M.A., Removal of carcinogenic hexavalent chromium from aqueous solutions using newly synthesized and characterized polypyrrole–titanium( IV) phosphate nanocomposite, Chem. Eng. J., 2015, vol. 280, pp. 494–504.
Trublet, M., Maslova, M.V., Rusanova, D., and Antzutkin, O.N., Mild syntheses and surface characterization of amorphous TiO(OH)(H2PO4) · H2O ionexchanger, Mater. Chem. Phys., 2016, vol. 183, pp. 467–475.
Khainakova, O.A., Espina, A., Trobajo, C., Khainakov, S.A., Garcia, J.R., and Bortun, A.I., Ionexchange properties of a novel layered titanium(IV) phosphate, Studies Surf. Sci. Catal., 2002, vol. 144, pp. 701–708.
Ivanenko, V.I., Lokshin, E.P., Aksenova, S.V., Korneikov, R.I., and Kalinnikov, V.T., Thermodynamics of cationic substitutions on titanyl hydrogen phosphate, Russ. J. Inorg. Chem., 2008, vol. 53, no. 4, pp. 557–563.
Maslova, M.V., Gerasimova, L.G., and Nikolaev, A.I., Sorption behavior of amorphous titanium phosphate for transition metal cations, Mezhdunarodn. Zh. Prikl. Fundament. Issledovanii, 2016, no. 4, pp. 356–361.
Bortun, A.I., Malinovskii, G.A., Khainakov, S.A., and Belyakov, V.N., Amphoteric properties of ion exchangers based on titanium and zirconium phosphates with low phosphorus content, Ukr. Khim. Zh. (Russ. Ed.), 1990, vol. 56, no. 1, pp. 7–10.
Lokshin, E.P., Ivanenko, V.I., Avsaragov, Kh.B., Mel’nik, N.A., Vladimirova, V.V., and Kalinnikov, V.T., Removal of radionuclides from aqueous salt solutions using titanium(IV) and zirconium(IV) phosphates, At. Energiya, 2002, vol. 92, no. 2, pp. 118–123.
Ivanenko, V.I., Lokshin, E.P., Udalova, I.A., Rosenbluh, M., Kaganovskii, Yu., Zdobina, S.V., and Kalinnikov, V.T., Synthesis of fine powders of KTP-group compounds of stoichiometric composition, Powder Technol., 2006, vol. 166, pp. 24–29.
Guan, J., Wang, L., Fu, G., and Li, Sh., Photoluminescence investigation of erbium-implanted in KTiOPO4 and RbTiOPO4, Nucl. Instrum. Methods Phys. Res., 2013, no. 307, pp. 482–485.
Mei, Y., Chen, B-W., Wei, Ch-F., and Zheng, W-Ch., Theoretical research of the spin-Hamiltonian parameters for two rhombic W5+ centers in KTiOPO4 (KTP) crystal through a two-mechanism model, Physica, 2016, no. 497, pp. 31–33.
Kopytina, A.V., German, K.E., Zhizhina, K.Yu., Zhukov, A.F., Ilyina, E.G., and Zhukova, T.V., Ion selective potentiometric sensor based on single crystalline KTiOPO4 for determination of K+-ions, Proc. Eng., 2016, no. 68, pp. 440–443.
Malekfar, R., Ahmadi, G., Cheraghi, A., Rohollahnejad, J., Sahraiyan, F., and Khanzadeh, M., Micro-Raman scattering of KTP (KTiOPO4) nanocrystallites synthesized by modified sol–gel Pechini method, Vibrat. Spectrosc., 2009, vol. 51, pp. 308–312.
Ivanenko, V.I., Lokshin, E.P., Korneikov, R.I., and Kalinnikov, V.T., Increase in the performance of titanium phosphate sorbents by modifying with transition metal cations, Dokl. Chem., 2011, vol. 439, no. 4, pp. 230–232.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © R.I. Korneikov, S.V. Aksenova, V.I. Ivanenko, E.P. Lokshin, 2018, published in Neorganicheskie Materialy, 2018, Vol. 54, No. 7, pp. 726–731.
Rights and permissions
About this article
Cite this article
Korneikov, R.I., Aksenova, S.V., Ivanenko, V.I. et al. Stability of Titanyl Hydrogen Phosphates in Aqueous Media. Inorg Mater 54, 689–693 (2018). https://doi.org/10.1134/S0020168518070063
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168518070063