Abstract
Cancer is one of the most common diseases worldwide, and its treatment is associated with many challenges such as drug and radioresistance and formation of metastases. These difficulties are due to tumor heterogeneity, which has many causes. One may be the cell fusion, a process that is relevant to both physiological (e.g., wound healing) and pathophysiological (cancer and viral infection) processes. This literature review aimed to summarize the existing data on the hybrid/atypical forms of circulating cancer cells and their role in tumor progression. For that, the bioinformatics search in universal databases, such as PubMed, NCBI, and Google Scholar was conducted by using the keywords “hybrid cancer cells”, “cancer cell fusion”, etc. In this review the latest information related to the hybrid tumor cells, theories of their genesis, characteristics of different variants with data from our own researches are presented. Many aspects of the hybrid cell research are still in their infancy. However, with the level of knowledge already accumulated, circulating hybrids such as CAML and CHC could be considered as promising biomarkers of cancerous tumors, and even more as a new approach to cancer treatment.
Similar content being viewed by others
INTRODUCTION
Currently, cancer is a leading cause of death in the majority of countries. There were 19.3 million of cancer cases in 2020 alone, of which approximately 10 million resulted in death [1]. In the Russian Federation, 591,371 cases of cancer, with 312,122 of deaths were registered [2], which put this disease in the second place based both on occurrence and mortality rates in the country [1]. High mortality rate is determined by numerous unique traits of this disease, including metastatic process [3] and chemoresistance [4], which are exceptionally important problems of oncology worldwide. It is already known that the fundamentally different group of cells – hybrid cells between the cancerous and healthy cells of the patient – plays a role in the abovementioned processes. In this review we will cover the latest information about the form of malignant cells, which recently received close attention of the scientists. The theories on formation of hybrids, their types and properties are discussed here together with information from our own research.
HISTORY OF HYBRID CELL RESEARCH
Cell fusion is the process opposite to cell division, which entails merging of cell membranes and intracellular components belonging to two cells. Osteoclasts, myocytes of skeletal muscles, and syncytiotrophoblast are commonly formed in a human organism under normal conditions via this mechanism. Cell fusion takes place during regeneration of internal organs. Thus, formation of hybrids is involved in several important processes in the human body [5].
In 1911 Otto Aichel suggested that cancerous cells are able to fuse with leukocytes, creating malignant hybrid cells [5]. His hypothesis was proven in 1974 by Goldenberg et al. [6]. Among the cells of human astrocytic glioma, which were grown inside a golden hamster, he found chromosomes, belonging to both donor and recipient. Hybrid cells have the features of both cancerous cells (unlimited growth and reproduction) and leukocytes (chemotaxis) [5]. Combination of those characteristics makes hybrids quite prone to formation of metastasis, which was observed by different researchers [7]. The opposite results reported by Harris et al. (1969) and (1989) should be noted [8, 9]. He showed that the fusion of normal mice fibroblasts with different lines of malignant cells led to formation of the stable hybrids, which had chromosomal biomarkers of both parent cells and did not grow into tumors in histocompatible animals. And despite the fact that currently available data suggest malignant nature of the hybrid cells [10-12], the study by Harris led to suggestion of existence of the tumor growth suppression genes.
THEORIES OF HYBRID GENESIS
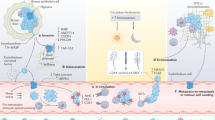
To date, numerous theories have been suggested on the hybrid genesis. The most well-researched fusion mechanism among them is the syncytin-dependent one (figure, a). In short, this process can be divided into three stages. The first one implies convergence of the membranes of both cells to the point when there are no water molecules left between the membranes. During that, rearrangement of the negatively charged phospholipids, including phosphatidylserine, from the inner to the outer layer of cell membrane takes place in the location of contact. Thus, the contents of both outer layers begin to mix with each other, indicating the beginning of the next stage of fusion – hemifusion. Finally, a fusion stalk is formed between the two cells. Its walls are composed of the combined bilipid layers from both cells. The stalk is filled with cytoplasm of the parent cells. The stalk widens, completing the fusion. During this process, the most important tasks such as cell convergence and initiation of fusion are performed by the specific transmembrane proteins – fusogenes, such as syncytin-1 and 2 [13]. Their role in cell hybridization was confirmed numerous times in multiple experiments. For example, in Mezler et al. (2018) work [14] the mesenchymal stem cells (MSC) and SK-OV-3 ovarian cancer cells were fused. These cells expressed syncytin-2 and MFSD-2A on their surface, which under normal condition appear on the syncytiotrophoblast of the healthy placenta. The genes responsible for syncytin synthesis were inserted into the eukaryotic genome by retroviruses millions of years ago. Hence, various viral infections [15], including SARS-CoV-2 [16], cause hybridization of cells. Some bacteria are also able to cause cell fusion [17].

Theories of cell fusion. a) Syncytin-dependent mechanism. 1) Convergence of cell membranes. 2) Hemifusion – mixing of lipids of the membrane outer layers. 3) Creation of fusion stalk. b) Direct interaction between cells accompanied by direct exchange of cell molecules. c) Indirect interaction with exchange of molecules between the distant cells. d) Acquiring of the cancer cell material by phagocytosis. e) Entosis – enveloping of a living cell by another one while maintaining its viability. f) Trogocytosis (left) – “biting” into a living cells acquiring properties of the donor cell. Transfer of the cytoplasmic elements acquired in this way to other cells is called dragocytosis (right). g) Exchange with the help of “tunnel nanotubes”
Other fusion theories include direct interaction theory (figure, b), describing exchange of RNA, growth factors, and cytokines between the cancer cells and MSCs. Indirect interaction theory (figure, c) explains hybrid genesis with a similar mechanism, but transmission of the molecules is realized via small vesicles, which are released by the cells into extracellular media. Phagocytosis theory implies acquiring of the cancer cell elements by leukocytes via phagocytosis (figure, d) [18], including its variations, such as entosis (figure, e) – a complete engulfing of one cell by another. During in vitro experiment with MSCs and embryonic stem cells the latter can envelope the mesenchymal stem cells with preservation of their viability [19]. Another variation of phagocytosis involved in the hybridization process is called trogocytosis (figure, f) – “biting” into the cancerous cell with further presentation of antigens belonging to the donor cell on the surface of the recipient cell. In most cases, the donor cell loses its functions by transferring them to the recipient cell, or dies. The cell death after trogocytosis is called trogoptosis [20]. Trogocytosis between the MSCs and ovarian cancer cells leading to chemoresistance was described [21]. It is possible that the material of malignant cells is passed to MSCs not via their direct contact with cancer cells, but by delivery from macrophages, which obtain the components of malignant cells via trogocytosis. This type of cell interaction is called dragocytosis (figure, f). Currently it has been confirmed that the specific bacteria species can survive via such mechanism [22]. It was suggested in the Manjunath et al. (2020) review [10] that the transfer of ions, molecules, vesicles, and organelles between the cells can be accomplished by formation of the so-called “tunnel nanotubes” (figure, g). These are thin, hollow structures from 20 to 500 nm in diameter, containing F-actin and microtubules, which are formed by cells under stress conditions, such as lack of nutrients, viral infections, oxidative stress, etc. Formation of such structures and their attachment to the surrounding cells helps them to survive adverse conditions. Perhaps this is how cancer cells connect with macrophages, which could result in fusion. Finally, the Notch-signaling and gap junction could also be involved in the fusion process [21].
After the fusion hybrid cells undergo the post-fusion (or hybrid) selection process (PHSP), which includes spontaneous hetero-to-synkaryon transitions (HST, transition from multinucleated cells to mononucleated cells), chromosomal rearrangements, and mitosis malfunctions. The PHSP is a quite unstable process, which is sensitive to external signals. Failure of the nucleus fusion or of rearrangement of genetic material leads to termination of the fusion process and cell death by apoptosis or necrosis. Because of that, emergence of the viable hybrid cell is a rare event, but in cases of successful selection the resulted cell receives the abilities of uncontrollable growth coupled with the mechanisms of escaping from cellular immunity and chemoresistance. Such hybrids are able to rapidly multiply and metastasize [23].
CHARACTERISTIC OF HYBRID CANCER CELLS
Throughout the study of hybrid cells multiple types of hybrid cells were identified. The main difference between them is the types of parent cells, which join in the course of the fusion process. The data on various types of hybrid cell populations are presented in table.
Characteristics of hybrid cell types
Type of hybrid cell | General characteristics | Role in cancer progression (+, stimulates; –, inhibits; ?, unknown) | References |
|---|---|---|---|
Osteoclasts | large, irregularly shaped multinucleate cells that facilitate bone remodeling; usually located in the zones of bone lysis/synthesis; they emerge as a result of successive merging of multiple small mononucleated preosteoclasts, one-by-one; thus, the cells increase their osteolytic activity (the same effect is possible after fusion with myeloma cells) | + | |
Syncytiotrophoblast | cytotrophoblast cells fuse with each other into structures with multiple elongated nuclei, well developed endocytotic mechanism, and multiple microvilli on the free cell surface; multinucleated syncytial structures facilitate exchange function of placenta and production of placental hormones; the multinucleated syncytiotrophoblast is maintained via continuous addition of mononucleated cytotrophoblasts | ? | |
Myofibers of skeletal muscles | a multinuclear structure of a cylindrical shape, reaching on average 10 cm in length; it develops as a result of the fusion of multiple myoblasts; the main functional unit of a skeletal muscle | ? | [29] |
“Cancer cell + healthy cell” hybrids | in most cases, morphology of the resulting cell is taken from the cancer parent cell; the surface markers are dependent on both precursor cells; hybrid cells demonstrate increased colony-forming abilities and therapy resistance (compared to parent cells); usually acquire properties of stem cells | + | |
“Cancer cell + fibroblast” hybrids | multinucleated cells with different morphology, exhibit low survival and proliferative activity: they cease to divide and die due to “mitotic catastrophe” in 97% of cases; however, survived cells acquire uniform morphology similar to the parental cancer cells, and begin to grow rapidly, while being resistant to classic treatment approaches | +/– | |
“Cancer cell + macrophage” hybrids | cancer-associated macrophage-like cells (CAML) and circulating hybrid cells (CHC) belongs to this group; the average size is 43.5 µm with oval, amorphous or tadpole-like shape; detected in most biological fluids of the patient by selecting CD45 and EpCAM markers; most of the hybrids have an increased metastatic potential and far more mobile under the effect of chemoattractants, compared to the parental cancer cells | + | |
“Cancer cell + lymphocyte” hybrids | represented here by hybridomas – artificially grown hybrids between cancerous and plasma cells, which are able to rapidly synthesize monoclonal antibodies in large quantities; presence of such hybrids in oncological patients was not confirmed | ? | [38] |
“Cancer cell + dendritic cell” hybrids | artificial, irregularly shaped multinucleated cells with short appendages; they are able to present cancer antigens to T-cells and stimulate activity of the cytotoxic lymphocytes better than the cancer or dendritic cells; considered as “cancer vaccines” | – | |
“Cancer cell + stem cell” hybrids | can retain the cancer cell morphology or take a fibroblast-like appearance; keep mesenchymal genetic and molecular profiles; surface markers vary and depend on the parent cells; hybrids display increased mobility, proliferative activity, and therapy resistance; it is assumed that the cancer stem cells (CSC) emerge as a result of such a fusion | + |
“Cancer cell + healthy cell” hybrids. This group is the most diverse in comparison with other types of hybrid cells [30-34, 43-45]. The possibility of the tumor cells fusion with epithelial cells [30-33, 45], fibroblasts [34, 43], and osteoclasts [44] has been described. Their only common traits include increased resistance to therapy and higher replication rates. Other characteristics, such as surface markers and typical genetic mutations depend on the parent cells. Inheritance of the stem cells characteristics from the parent cancer cell is often observed, such as appearance of stem cells transcriptional factors – Oct4, Sox2, Nanog, Kif4, Bmi1, and CD133, and increase of metastatic potential with mammosphere formation in breast cancer. In the majority of cases, morphology of the parental cancer cell is also retained by the hybrids. In multiple experiments [31, 32] on fusion of breast epithelial cells with different breast cancer cell lines each formed hybrid showed different capabilities of growth and reproduction. Thus, it is very likely that the hybrid cells contribute significantly to cancer heterogeneity.
Clinical importance of hybrid cells identification is based on the differences in activity of biochemical processes and signaling pathways from conventional malignant cells. For example, the cells produced by fusion with two different breast cancer cell lines exhibited an altered RAF-AKT pathway activity. This led to paradoxical reaction of the hybrids to PI3K inhibitor – administration of this drug caused significant increase in the malignant cells mobility, while the response of original cancer cells involved migration blockage. This shows importance of detection of hybrid cells in the patients for selection of effective therapy [45].
“Cancer cell + stem cell” hybrids. Hybrids of cancer cells with stem cells are also quite diverse [11, 41, 42, 46-49], similarly to the hybrids from the previous group. There is an assumption that hybridization of cancer cells with MSCs or macrophages plays a crucial role in metastatic process, since cancer cells acquire the stem cell properties and become able to circulate in the bloodstream. It was shown in Li et al. (2019) experiments [46] that after the fusion of cancer cells with the omental adipose-derived stromal cells (O-ASCs), which are similar in their characteristics to MSCs, the resulting hybrid cells exhibited enhanced mobility in comparison with their parental malignant cells. The authors suggested that it was caused by the decrease in E-cadherin and increase in vimentin expressions. That was also confirmed in other experiments [41, 42], where after the fusion of cancerous and stem cells the formed hybrids went through epithelial-mesenchymal transition (EMT) leading to the increase of their proliferative and migratory capabilities. In the process, the newly formed hybrids can either retain morphology of the cancerous epithelial cell [42, 50] or change it to more mesenchymal, fibroblast-like appearance [51], but in all cases the hybrids have mesenchymal genetic and molecular profiles. The observed tendency of hybrids to aneu- and polyploidy and detection of this phenomenon in metastatic cells, as well as the fact that the emergence of tetraploids in non-hybrid malignant cells is not common, further confirms this theory [42].
Probability of the genesis of cancer stem cells (CSCs) through fusion of cancer and stem cells cannot be ruled out. CSCs is a subpopulation of cancer cells with low proliferative potential, but exceptionally high survivability. This is facilitated by their insensitivity to apoptotic signals and resistance to anti-cancer drugs. They are playing the role of “sleeping cells”, ready to start multiplying even after total eradication of the primary tumor thus causing cancer recurrence. It was shown in numerous studies that hybridization of MSCs and cancer cells produces hybrids with the traits of CSCs. The hybrid theory could explain emergence of this not well-known cell subpopulation, but further research to confirm this hypothesis is required [5].
“Cancer cell + macrophage” hybrids. Despite the increased metastatic potential of all abovementioned hybrids, there is no available data confirming their circulation in blood and other biological fluids, which makes challenging their detection and severely diminishes their diagnostic significance. Instead, a more homogeneous type of hybrid cells can be found in biological fluids – fusions of cancer cells with macrophages. Currently it can be stated that these hybrids represent the most popular object in the hybrid cell research. Several subtypes of such hybrids have already been described, but their similarity should be noted. There is a probability that scientists gave different unique names to the same type of cells. In particular, in 2014 Adams et al. [52] described the cancer-associated macrophage-like cells, or CAMLs in blood of the patients with breast and pancreatic cancer. Other authors [10] gave the name “Macrophage-Tumor cell Fusion cells” (MTF) to the similar cells. Morphologically they are highly differentiated myeloid cells capable of phagocytosis, with big atypical nucleus or with multiple smaller nuclei [52]. The size of CAMLs varies from 21 to 300 µm with average size of 43.5 µm, while the circulating tumor cells (CTC) and leukocytes have sizes of 18.8 and 12.4 µm, respectively [35]. CAMLs are able to take an oval, amorphous, or tadpole-like shape [36]. These cells are present in the blood of patients with a broad spectrum of oncological diseases (8.2 cells/7.5 ml) and sometimes in significantly greater quantities such as in ascitic fluid (~600.000 cells/ml in ovarian cancer patients), and do not appear in the healthy people [53]. CAMLs originate from the tissues of the primary tumor – up to 13% of all cells [54]. These cells express CD45, which is common for every human leukocyte [55], EpCAM (epithelial cell adhesion molecule), which is found in most epithelial cancer cells [56], and cytokeratins [57]. Kaigorodova et al. (2020) [58] identified 12 different populations of cancer cells in the ascites fluid from ovarian cancer patients. The authors found that the majority of cells were represented by atypical cells with hybrid phenotype, stemness, and evidences of EMT (EpCAM+, CD45+, CD44+, CD24+/–, CD133+/–, N-cadherin+/–). This information was repeatedly confirmed. It was also shown in our studies that the number of atypical/hybrid forms of EpCAM+CD45+ cancer cells in ascitic fluid of patients diagnosed with ovarian cancer has a direct correlation with the degree of carcinomatosis [59]. Sukhbaatar et al. (2017) also found EpCAM+CD45+ cells during the search for TP53 mutations in the ovarian cancer cells, but could not find any mutations in this gene in them. The authors showed from 10 to 170-fold increase in the expression of ZEB1, SNAIL, TWIST, INMB1, THNSB, and COL11 genes in CAML, which resulted in the increased amounts of N-cadherin and vimentin in these cells [60].
In addition to CAMLs, small (5-20 µm) round cells with 1-2 round nuclei inside and markers identical to CAML have been detected. These cells were called “circulating hybrid cells” or CHCs. They were also detected in the blood of oncological patients in numbers far larger than the quantities of CTCs – on average approximately 5-70 cells per 500,000 live cells depending on the type of oncological disease, up to thousands in the cases of uveal melanoma [61]. It was hypothesized that CHCs are the products of cell fusion, when CAMLs are produced as a result of engulfing of cancer cells [57]. A couple of works proves that the metastatic potential of CHCs (in particular, it was shown in the study by Gast et al. (2018) [12]) that the fusion of macrophages with adenocarcinoma and melanoma cells leads to emergence of the hybrid cells with increased proliferative and metastatic potentials. Interestingly, the cells stop their reproduction over time under in vitro conditions. The increased hybrids proliferation was detected in vivo, which implies positive connection between the “cancer cell + macrophage” hybrids and signals from microenvironment. Even more curious is the fact that CHCs are present even in the blood of healthy individuals, what fundamentally differentiate them from CAML [7]. Heterogeneity of CHCs in expression of the genes responsible for mesenchymal (vimentin, E-cadherin), stem properties (CD44, CD133, ALDH1), and some receptors (androgen receptor, AR) has been reported. Only CHCs with strong mesenchymal and stem properties are involved in metastatic process, and the increased AR expression in breast cancer indicates increased resistance to therapy [62-64].
Cells with CD163 [65-67], CD204, and CD206 [68] were detected repeatedly among the “cancer cell + macrophage” hybrids. These markers are specific to M2-macrophages. Contrary to the pro-inflammatory M1-macrophages, M2-cells inhibit inflammation and stimulate the processes of cancer growth and progression. It was found that only after the fusion of a cancer cell with an M2-macrophage the resulting hybrid acquires the properties of stem cell and macrophage, thus obtaining increased metastatic capabilities [69]. More than that, a selective fusion of M2-macrophages with cancer cells has been observed [70]. The “cancer cell + macrophage” hybrids are likely to originate from the fusion with tumor-associated macrophages (TAMs) – macrophages in the composition of solid tumors that maintain its viability. They have a M2-like phenotype, and it has been suggested that TAMs and M2-macrophages are the same cells, though the latest research speaks the opposite [71].
The “cancer cell + macrophage” hybrids attract an exceptionally high interest of the scientific community due to their presence in blood in significant quantities even at the earliest stages of the disease [10] and ease of obtaining. Potentially it makes them an efficient and reliable biomarker of solid tumors. Enormous size of CAMLs significantly simplifies the task of isolation of these hybrids, allowing to use technically simple methods, such as microfiltration [35].
In the recent studies it has been suggested that the EpCAM+CD45+ cells exhibit increased malignancy and chemotherapy resistance. In particular, it is considered that these cells excessively express HLA I and HLA II on their surface, which helps them to escape NK-cells. Regarding chemoresistance, CAMLs show increased resistance to, for example, cisplatin and paclitaxel (more than 27 µg/ml, comparing to 8-12 and 7-10 for CD45–EpCAM+ cells, respectively) [52, 72, 73], and to radiotherapy due to elevated DNA repair capabilities [66, 74].
The fact of tumor malignancy itself could be established by the presence of CAMLs. In Adams et al. (2016) research [75] these hybrids were detected in 88% of the patients with invasive breast carcinoma, and only in 26% – with benign growths. Many authors consider direct correlation between the concentration of hybrid cells and patient’s outcome. But, despite the significant increase in CD45+EpCAM+ cells concentrations in the latter stages of disease, their presence was also observed in the earlier stages [57]. Presence of these cells long before the development of metastases was described, which likely makes them a precursor of the tumor upcoming dissemination [10]. Thus, in our latest research it was shown that the atypical/hybrid forms of EpCAM+CD45+ cancer cells could already be detected in the blood of the patients with endometrial cancer at T1 stage. Their number correlates with the risk of recurrence after treatment [76]. Based on examination of the patients with esophagus [35], lung [77], pancreatic [78], and other cancers [53], it was revealed that the CAML size of ≥50 µg and their concentration in blood of ≥5-6 cell/7.5 ml is associated with low survivability and high risk of recurrence after therapy. During the simultaneous analysis of CAML and CTCs levels it was demonstrated that the patients with increased amounts of both types of cells in blood (≥5 CTCs/7.5 ml) had lower chances of survival in comparison with the patients with only elevated levels of CTCs [79].
CHCs themselves also serve as valuable biomarkers in cancer diagnostics and monitoring, not only due to their high concentrations in the patient’s blood [80-82]. It was established in different studies of the patients with various gastrointestinal cancers [80, 82] that there was a decrease in the levels of hybrids in blood after chemotherapy. Increase of the CHCs concentration in blood of the patients with oral carcinomas allowed Henn et al. (2021) predicting the emergence of hidden metastases in lymph nodes, thus providing an opportunity to avoid the highly invasive neck dissection, which leads to complications in 25% of the cases [81].
An important problem on the path of CHCs to the status of clinical biomarker is their similarity with normal leukocytes of the patient. Because of that, a fast, simple, and efficient isolation of these hybrids and their following analysis is complicated. One of the developing methods for overcoming this issue is dielectrophoretic separation in a specifically designed microfluidic chip. Separation of the cells occurs due to their differences in polarization of each cell and forces of the fluid flow. In the experiment with blood samples from the patients with pancreatic adenocarcinoma the possibility to increase concentration of CHCs 18.6-fold by excluding 96.5% of leukocytes from the sample was demonstrated [83].
HYBRID CELLS AND CANCER THERAPY
Evidence of the role of hybrids in cancer spread and drug resistance shows the possibility of developing efficient cancer treatment strategies targeting hybrid cells. The most obvious approach here seems to be blocking the fusion process itself. It is known that hypoxia and TNFα stimulate cell fusion [84]. They are also involved in apoptosis induction. Scientists confirmed the role of this type of programmed cell death in emergence of hybrids. Hence, it seems possible to suppress cell fusion through inhibition of one or another element of the signaling pathways involved in apoptosis. There is a sufficient amount of research in this field. For example, it is possible to disrupt the TNFα receptor function by siRNA [85] or minocycline antibiotic [86]. The well-known and actively developing method of halting TNFα synthesis from its precursor pro-TNFα is inhibition of the ADAM17 enzyme [87]. Creation of monoclonal antibodies targeting TNFα or apoptotic pathways is also quite popular [88]. Other targets for apoptosis inhibition are phosphatidylserine [89], MMP9 [90], caspases [91], etc. Some of the described here approaches for anti-TNFα-therapy appeared in the arsenals of oncologists long ago, but evaluation of the role of this factor in cell fusion could increase their acceptance among doctors.
The role of stimulating factors was also proven by the fact that they are characteristic for inflammation that accompanies both tissue regeneration and cancer progression. In both cases, cell fusion is present [92]. Hence, the anti-inflammatory therapy could also help in stopping hybridization of cancer cells as was demonstrated, for example, in the experiment conducted by Li et al. (2013) [93], where successful blocking of IL-4 receptors in the rhabdomyosarcoma cells prevented cancer growth and progression in vivo.
Another technique of cell fusion prevention is by disrupting syncytins’ function. Different authors used antisense-nucleotides and small hairpin RNA for inhibition of the syncytin-1 and 2 genes expression, respectively. At the same time, association between the syncytin expression and positive outcome for the patients with breast cancer was reported, thus, relevance of such therapeutic approach requires additional research [94].
Naturally, if something stimulates cell fusion, physiological inhibitors of hybridization also should exist. It was shown that TGFβ in large quantities suppresses cell fusion, but it could not be considered as an anti-cancer drug due to its effect of stimulation of cell proliferation, including malignant ones [95].
The hybrids between cancer and dendritic cells (DCs) should be covered here separately [40, 96, 97]. These are artificially created cells with antigens belonging to cancer and antigen-presenting cells simultaneously. This allows such hybrids to effectively stimulate the patient’s own anti-cancer immune system. The possibility of using the hybrids of a cancer cell with a DC was described long ago [40]. Numerous methods using such cells in cancer treatment are currently under development. One of such approach is based on the zirconium metal-organic frameworks nanoparticles, which are commonly used for photodynamic therapy, coated by the hybrid cell membranes. After their injection into the bloodstream of a patient, they accumulate in tumors via the enhanced permeability and retention effect (EPR) and presence of cancer cell proteins on the nanoparticle surface. The metal-organic framework nanoparticles generate a significant amount of reactive oxygen species under the laser irradiation, and antigens from the cancer and dendritic cells on the nanoparticle surface stimulate strong immune response from T-killers. In vivo experiments with mice with inoculated and grown tumors showed a considerable reduction in the tumor growth rates on the 28th day after the start of the therapy in comparison with the effect of other nanoparticles used for photodynamic therapy. On the 62th day 40%-survival among the animals in the “nanoparticle + hybrid cell membrane” experiment group was achieved, while in other groups all the mice died [97].
CONCLUSIONS
Despite the fact that the phenomenon of cell fusion was discovered more than 100 years ago, all this time it was mostly out of the sight of researchers – all the attention of the scientific community was on the CSCs theory. That is why many aspects of the hybrid cell research are still in the early stages of development. Nevertheless, even at the present level of knowledge, the circulating hybrids, such as CAML and CHCs, are considered as promising biomarkers of cancer, and even more – as a new approach to cancer therapy. Emergence of such novel techniques indicates that there is a large, uncovered potential of hybrid cells requiring extensive research.
Abbreviations
- CAML:
-
cancer-associated macrophage-like cells
- CHC:
-
circulating hybrid cells
- CSC:
-
cancer stem cell
- CTC:
-
circulating tumor cells
- MSC:
-
mesenchymal stem cells
References
Sung, H. (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA: A Cancer J. Clinic., 71, 209-249, https://doi.org/10.3322/caac.21660.
Russian Federation. Cancer Country Profile 2020, URL: https://www.who.int/cancer/country-profiles/RUS_2020.pdf.
Chaffer, C. L., and Weinberg, R. A. (2011) A perspective on cancer cell metastasis, Science, 331, 1559-1564, https://doi.org/10.1126/science.1203543.
Kachalaki, S., Ebrahimi, M., Mohamed Khosroshahi, L., Mohammadinejad, S., and Baradaran, B. (2016) Cancer chemoresistance; biochemical and molecular aspects: A brief overview, Eur. J. Pharm. Sci., 89, 20-30, https://doi.org/10.1016/j.ejps.2016.03.025.
Shabo, I., Svanvik, J., Lindström, A., Lechertier, T., Trabulo, S., et al. (2020) Roles of cell fusion, hybridization and polyploid cell formation in cancer metastasis, J. Clin. Oncol., 11, 121-135, https://doi.org/10.5306/wjco.v11.i3.121.
Goldenberg, D. M., Pavia, R. A., and Tsao, M. C. (1974) In vivo hybridisation of human tumour and normal hamster cells, Nature, 250, 649-651, https://doi.org/10.1038/250649a0.
Reduzzi, C., Vismara, M., Gerratana, L., Silvestri, M., De Braud, F., et al. (2020) The curious phenomenon of dual-positive circulating cells: Longtime overlooked tumor cells, Semin. Cancer Biol., 60, 344-350, https://doi.org/10.1016/j.semcancer.2019.10.008.
Harris, H. (1989) The biology of tumour suppression, CIBA Found. Symp., 142, 199-208.
Harris, H., Miller, O. J., Klein, G., Worst, P., and Tachibana, T. (1969) Suppression of malignancy by cell fusion, Nature, 223, 363-368.
Manjunath, Y., Porciani, D., Mitchem, J. B., Suvilesh, K. N., Avella, D. M., et al. (2020) Tumor-cell-macrophage fusion cells as liquid biomarkers and tumor enhancers in cancer, Int. J. Molecular Sci., 21, 1872, https://doi.org/10.3390/ijms21051872.
Pawelek, J. M., and Chakraborty, A. K. (2008) Fusion of tumour cells with bone marrow-derived cells: A unifying explanation for metastasis, Nat. Rev. Cancer, 8, 377-386, https://doi.org/10.1038/nrc2371.
Gast, C. E., Silk, A. D., Zarour, L., Riegler, L., Burkhart, J. G., et al. (2018) Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival, Sci. Adv., 4, eaat7828, https://doi.org/10.1126/sciadv.aat7828.
Bastida-Ruiz, D., Van Hoesen, K., and Cohen, M. (2016) The dark side of cell fusion, Int. J. Mol. Sci., 17, 638, https://doi.org/10.3390/ijms17050638.
Melzer, C., von der Ohe, J., and Hass, R. (2018) MSC stimulate ovarian tumor growth during intercellular communication but reduce tumorigenicity after fusion with ovarian cancer cells, Cell Commun. Signal., 16, 67, https://doi.org/10.1186/s12964-018-0279-1.
Podbilewicz, B. (2014) Virus and cell fusion mechanisms, Ann. Rev. Cell Dev. Biol., 30, 111-139, https://doi.org/10.1146/annurev-cellbio-101512-122422.
Wang, X., Chen, C. H., Badeti, S., Cho, J. H., Naghizadeh, A., et al. (2021) Deletion of ER-retention motif on SARS-CoV-2 spike protein reduces cell hybrid during cell-cell fusion, Cell Biosci., 11, 114, https://doi.org/10.1186/s13578-021-00626-0.
Ku, J. W. K., Chen, Y., Lim, B. J. W., Gasser, S., Crasta, K. C., and Gan, Y.-H. (2020) Bacterial-induced cell fusion is a danger signal triggering cGAS–STING pathway via micronuclei formation, Proc. Natl. Acad. Sci. USA, 117, 15923-15934, https://doi.org/10.1073/pnas.2006908117.
Broncy, L., and Paterlini-Bréchot, P. (2018) Cancer-associated circulating atypical cells with both epithelial and macrophage-specific markers, J. Lab. Prec. Med., 3, 91, https://doi.org/10.21037/jlpm.2018.10.05.
Sottile, F., Aulicino, F., Theka, I., and Cosma, M. P. (2016) Mesenchymal stem cells generate distinct functional hybrids in vitro via cell fusion or entosis, Sci. Rep., 6, 36863, https://doi.org/10.1038/srep36863.
Miyake, K., and Karasuyama, H. (2021) The role of trogocytosis in the modulation of immune cell functions, Cells, 10, 1255, https://doi.org/10.3390/cells10051255.
Melzer, C., Yang, Y., and Hass, R. (2016) Interaction of MSC with tumor cells, Cell Commun. Signal., 14, 20, https://doi.org/10.1186/s12964-016-0143-0.
Dragotakes, Q., Fu, M. S., and Casadevall, A. (2019) Dragotcytosis: Elucidation of the mechanism for cryptococcus neoformans macrophage-to-macrophage transfer, J. Immunol., 202, 2661-2670, https://doi.org/10.4049/jimmunol.1801118.
Melzer, C., Ohe, J. V., and Hass, R. (2020) Altered Tumor plasticity after different cancer cell fusions with MSC, Int. J. Mol. Sci., 21, 8347, https://doi.org/10.3390/ijms21218347.
Soe, K. (2020) Osteoclast fusion: Physiological regulation of multinucleation through heterogeneity-potential implications for drug sensitivity, Int. J. Mol. Sci., 21, 7717, https://doi.org/10.3390/ijms21207717.
Jacome-Galarza, C. E., Percin, G. I., Muller, J. T., Mass, E., Lazarov, T., et al. (2019) Developmental origin, functional maintenance and genetic rescue of osteoclasts, Nature, 568, 541-545, https://doi.org/10.1038/s41586-019-1105-7.
Andersen, T. L., Boissy, P., Sondergaard, T. E., Kupisiewicz, K., Plesner, T., et al. (2007) Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: A new type of cancer-host partnership, J. Pathol., 211, 10-17, https://doi.org/10.1002/path.2078.
Smirnova, T. L. (2009) Placenta. Stages of development. Bulletin of the Chuvash University, 2, 73-79.
Huppertz, B., and Gauster, M. (2011) Trophoblast fusion, Adv. Exp. Med. Biol., 713, 81-95, https://doi.org/10.1007/978-94-007-0763-4_6.
Cretoiu, D., Pavelescu, L., Duica, F., Radu, M., Suciu, N., et al. (2018) Myofibers, Muscle Atrophy, 23-46, https://doi.org/10.1007/978-981-13-1435-3_2.
Dittmar, T., Schwitalla, S., Seidel, J., Haverkampf, S., Reith, G., et al. (2010) Characterization of hybrid cells derived from spontaneous fusion events between breast epithelial cells exhibiting stem-like characteristics and breast cancer cells, Clin. Exp. Metastas., 28, 75-90, https://doi.org/10.1007/s10585-010-9359-3.
Fahlbusch, S. S., Keil, S., Epplen, J. T., Zänker, K. S., and Dittmar, T. (2020) Comparison of hybrid clones derived from human breast epithelial cells and three different cancer cell lines regarding in vitro cancer stem/initiating cell properties, BMC Cancer, 20, 446, https://doi.org/10.1186/s12885-020-06952-9.
Gauck, D., Keil, S., Niggemann, B., Zänker, K. S., and Dittmar, T. (2017) Hybrid clone cells derived from human breast epithelial cells and human breast cancer cells exhibit properties of cancer stem/initiating cells, BMC Cancer, 17, 515, https://doi.org/10.1186/s12885-017-3509-9.
Berndt, B., Haverkampf, S., Reith, G., Keil, S., Niggemann, B., et al. (2013) Fusion of CCL21 non-migratory active breast epithelial and breast cancer cells give rise to CCL21 migratory active tumor hybrid cell lines, PLoS One, 8, e63711, https://doi.org/10.1371/journal.pone.0063711.
Wang, R., Sun, X., Wang, C. Y., Hu, P., Chu, C.-Y., Liu, S., et al. (2012) Spontaneous cancer-stromal cell fusion as a mechanism of prostate cancer androgen-independent progression, PLoS One, 7, e42653, https://doi.org/10.1371/journal.pone.0042653.
Gironda, D. J., Adams, D. L., He, J., Xu, T., Gao, H., et al. (2020) Cancer associated macrophage-like cells and prognosis of esophageal cancer after chemoradiation therapy, J. Transl. Med., 18, 413, https://doi.org/10.1186/s12967-020-02563-x.
Mu, Z., Wang, C., Ye, Z., Rossi, G., Sun, C., et al. (2017) Prognostic values of cancer associated macrophage-like cells (CAML) enumeration in metastatic breast cancer, Breast Cancer Res. Treat., 165, 733-741, https://doi.org/10.1007/s10549-017-4372-8.
Rachkovsky, M., Sodi, S., Chakraborty, A., Avissar, Y., Bolognia, J., et al. (1998) Melanoma × macrophage hybrids with enhanced metastatic potential, Clin. Exp. Metastasis, 16, 299-312, https://doi.org/10.1023/a:1006557228604.
Kohler, G., and Milstein, C. (1975) Continuous cultures of fused cells secreting antibody of predefined specificity, Nature, 256, 495-497.
Koido, S., Ohana, M., Liu, C., Nikrui, N., Durfee, J., et al. (2004) Dendritic cells fused with human cancer cells: Morphology, antigen expression, and T cell stimulation, Clin. Immunol., 113, 261-269, https://doi.org/10.1016/j.clim.2004.08.004.
Serhal, K., Baillou, C., Ghinea, N., Fontanges, P., Dupuy, F. P., et al. (2007) Characteristics of hybrid cells obtained by dendritic cell/tumour cell fusion in a T-47D breast cancer cell line model indicate their potential as anti-tumour vaccines, Int. J. Oncol., 31, 1357-1365, https://doi.org/10.3892/ijo.31.6.1357.
Xue, J., Zhu, Y., Sun, Z., Ji, R., Zhang, X., et al. (2015) Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness, BMC Cancer, 15, 793, https://doi.org/10.1186/s12885-015-1780-1.
Rappa, G., Mercapide, J., and Lorico, A. (2012) Spontaneous formation of tumorigenic hybrids between breast cancer and multipotent stromal cells is a source of tumor heterogeneity, Am. J. Pathol., 180, 2504-2515, https://doi.org/10.1016/j.ajpath.2012.02.020.
Kemény, L., Kurgyis, Z., Buknicz, T., Groma, G., Jakab, Á., et al. (2016) Melanoma cells can adopt the phenotype of stromal fibroblasts and macrophages by spontaneous cell fusion in vitro, Int. J. Mol. Sci., 17, 826, https://doi.org/10.3390/ijms17060826.
Hass, R., von der Ohe, J., and Dittmar, T. (2021) Hybrid formation and fusion of cancer cells in vitro and in vivo, Cancers, 13, 4496, https://doi.org/10.3390/cancers13174496.
Özel, C., Seidel, J., Meyer-Staeckling, S., Brandt, B. H., Niggemann, B., et al. (2012) Hybrid cells derived from breast epithelial cell/breast cancer cell fusion events show a differential RAF-AKT crosstalk, Cell Commun. Signal, 10, 10, https://doi.org/10.1186/1478-811X-10-10.
Li, M., Li, X., Zhao, L., Zhou, J., Cheng, Y., et al. (2019) Spontaneous formation of tumorigenic hybrids between human omental adipose-derived stromal cells and endometrial cancer cells increased motility and heterogeneity of cancer cells, Cell Cycle, 18, 320-332, https://doi.org/10.1080/15384101.2019.1568743.
Xu, M. H., Gao, X., Luo, D., Zhou, X. D., Xiong, W., et al. (2014) EMT and acquisition of stem cell-like properties are involved in spontaneous formation of tumorigenic hybrids between lung cancer and bone marrow-derived mesenchymal stem cells, PLoS One, 9, e87893, https://doi.org/10.1371/journal.pone.0087893.
Dörnen, J., Myklebost, O., and Dittmar, T. (2020) Cell fusion of mesenchymal stem/stromal cells and breast cancer cells leads to the formation of hybrid cells exhibiting diverse and individual (stem cell) characteristics, Int. J. Mol. Sci., 21, 9636, https://doi.org/10.3390/ijms21249636.
Li, H., Feng, Z., Tsang, T. C., Tang, T., Jia, X., et al. (2014) Fusion of HepG2 cells with mesenchymal stem cells increases cancer‑associated and malignant properties: An in vivo metastasis model, Oncol. Rep., 32, 539-547, https://doi.org/10.3892/or.2014.3264.
He, X., Li, B., Shao, Y., Zhao, Na, Hsu, Y., et al. (2015) Cell fusion between gastric epithelial cells and mesenchymal stem cells results in epithelial-to-mesenchymal transition and malignant transformation, BMC Cancer, 15, 24, https://doi.org/10.1186/s12885-015-1027-1.
Zhang, L. N., Kong, C. F., Zhao, D., Cong, X. L., Wang, S. S., et al. (2019) Fusion with mesenchymal stem cells differentially affects tumorigenic and metastatic abilities of lung cancer cells, J. Cell Physiol., 234, 3570-3582, https://doi.org/10.1002/jcp.27011.
Adams, D. L., Martin, S. S., Alpaugh, R. K., Charpentier, M., Tsai, S., et al. (2014) Circulating giant macrophages as a potential biomarker of solid tumors, Proc. Natl. Acad. Sci. USA, 111, 3514-3519, https://doi.org/10.1073/pnas.1320198111.
Adams, D., Adams, D. K., Lin, S. H., Cristofanilli, M., Bergan, R. C., et al. (2017) Cancer-associated macrophage-like cells as prognostic indicators of overall survival in a variety of solid malignancies, J. Clin. Oncol., 35, 11503-11503.
Akhter, M. Z., Sharawat, S. K., Kumar, V., Kochat, V., Equbal, V., et al. (2018) Aggressive serous epithelial ovarian cancer is potentially propagated by EpCAM+CD45+ phenotype, Oncogene, 37, 2089-2103, https://doi.org/10.1038/s41388-017-0106-y.
Rheinländer, A., Schraven, B., and Bommhardt, U. (2018) CD45 in human physiology and clinical medicine, Immunol. Lett., 196, 22-32, https://doi.org/10.1016/j.imlet.2018.01.009.
Huang, L., Yang, Y., Yang, F., Liu, S., Zhu, Z., et al. (2018) Functions of EpCAM in physiological processes and diseases (review), Int. J. Mol. Med., 42, 1771-1785, https://doi.org/10.3892/ijmm.2018.3764.
Sutton, T. L., Walker, B. S., and Wong, M. H. (2019) Circulating hybrid cells join the fray of circulating cellular biomarkers, Cell. Mol. Gastroenterol. Hepatol., 8, 595-607, https://doi.org/10.1016/j.jcmgh.2019.07.002.
Kaigorodova, E. V., Fedulova, N. V., Ochirov, M. O., Dyakov, D. A., Molchanov, S. V., et al. (2020) Dissimilar tumor cell populations in ascitic fluid of ovarian cancer patients, Bull. Siber. Med., 19, 50-58, https://doi.org/10.20538/1682-0363-2020-1-50-58.
Kaigorodova, E. V., Ochirov, M. O., Molchanov, S. V., Rogachev, R. R., Dyakov, D. A., et al. (2021) Dissimilar populations of EpCam-positive cells in ascitic fluid of ovarian cancer patients: A relationship with the degree of carcinomatosis, Bull. Siber. Med., 20, 44-53, https://doi.org/10.20538/1682-0363-2021-2-44-53.
Sukhbaatar, N., Bachmayr-Heyda, A., Auer, K., Aust, S., Deycmar, S., et al. (2017) Two different, mutually exclusively distributed, TP53 mutations in ovarian and peritoneal tumor tissues of a serous ovarian cancer patient: Indicative for tumor origin? Cold Spring Harb. Mol. Case Studies, 3, a001461, https://doi.org/10.1101/mcs.a001461.
Dietz, M. S., Sutton, T. L., Walker, B. S., Gast, C. E., Zarour, L., et al. (2021) Relevance of circulating hybrid cells as a non-invasive biomarker for myriad solid tumors, Sci. Rep., 11, 13630, https://doi.org/10.1038/s41598-021-93053-7.
Walker, B. S., Sengupta, S., Parappilly, M., Ors, A., Fischer, J., et al. (2020) Harnessing the heterogeneity of circulating hybrid cells in pancreatic adenocarcinoma, Proceedings of the AACR Virtual Special Conference on Tumor Heterogeneity: From Single Cells to Clinical Impact, Cancer Res., 80 (Suppl 21), PO-014, https://doi.org/10.1158/1538-7445.TUMHET2020-PO-014.
Dietz, M. S., Sutton, T., Walker, B., Chang, Y. H., Chin, K., et al. (2020) A novel disseminated tumor cell identified in myriad cancer harbors tumor initiating properties, Cancer Res., 80 (Suppl. 21), PO-102, https://doi.org/10.1158/1538-7445.TUMHET2020-PO-102.
Denisov, E. V., Menyailo, M. E., Zolotareva, S. Y., Gerashchenko, T. S., Alifanov, V. V., et al. (2021) 23P Transcriptional profiling of circulating tumor and hybrid cells in breast cancer patients, Ann. Oncol., 32 (Suppl. 5), S367-S368, https://doi.org/10.1016/j.annonc.2021.08.301.
Aljabery, F., Olsson, H., Gimm, O., Jahnson, S., and Shabo, I. (2018) M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer, Urol. Oncol. Semin. Origin. Invest., 36, 159.e19-159.e26, https://doi.org/10.1016/j.urolonc.2017.11.020.
Garvin, S., Oda, H., Arnesson, L. G., Lindström, A., and Shabo, I. (2018) Tumor cell expression of CD163 is associated to postoperative radiotherapy and poor prognosis in patients with breast cancer treated with breast-conserving surgery, J. Cancer Res. Clin. Oncol., 144, 1253-1263, https://doi.org/10.1007/s00432-018-2646-0.
Garvin, S., Vikhe Patil, E., Arnesson, L. G., Oda, H., Hedayati, E., et al. (2019) Differences in intra-tumoral macrophage infiltration and radiotherapy response among intrinsic subtypes in pT1-T2 breast cancers treated with breast-conserving surgery, Virch. Arch. Int. J. Pathol., 475, 151-162, https://doi.org/10.1007/s00428-019-02563-3.
Clawson, G. A., Matters, G. L., Xin, P., McGovern, C., Wafula, E., et al. (2017) “Stealth dissemination” of macrophage-tumor cell fusions cultured from blood of patients with pancreatic ductal adenocarcinoma, PLoS One, 12, e0184451, https://doi.org/10.1371/journal.pone.0184451.
Tretyakova, M. S., Subbalakshmi, A. R., Menyailo, M. E., Jolly, M. K., and Denisov, E. (2021) Tumor hybrid cells: Nature and biological significance, Preprints, 2021, 2021110250, https://doi.org/10.20944/preprints202111.0250.v1.
Aguirre, L. A., Montalbán-Hernández, K., Avendaño-Ortiz, J., Marín, E., Lozano, R., Toledano, V., et al. (2020) Tumor stem cells fuse with monocytes to form highly invasive tumor-hybrid cells, Oncoimmunology, 9, 1773204, https://doi.org/10.1080/2162402X.2020.1773204.
Zhou, J., Tang, Z., Gao, S., Li, C., Feng, Y., and Zhou, X. (2020) Tumor-associated macrophages: Recent insights and therapies, Front. Oncol., 10, 188, https://doi.org/10.3389/fonc.2020.00188.
Gubbels, J. A., Felder, M., Horibata, S., Belisle, J. A., Kapur, A., et al. (2010) MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells, Mol. Cancer, 9, 11, https://doi.org/10.1186/1476-4598-9-11.
Baligar, P., Mukherjee, S., Kochat, V., Rastogi, A., and Mukhopadhyay, A. (2016) Molecular and cellular functions distinguish superior therapeutic efficiency of bone marrow CD45 cells over mesenchymal stem cells in liver cirrhosis, Stem Cells, 34, 135-147, https://doi.org/10.1002/stem.2210.
Lindström, A., Midtbö, K., Arnesson, L. G., Garvin, S., and Shabo, I. (2017) Fusion between M2-macrophages and cancer cells results in a subpopulation of radioresistant cells with enhanced DNA-repair capacity, Oncotarget, 8, 51370-51386, https://doi.org/10.18632/oncotarget.17986.
Adams, D. L., Adams, D. K., Alpaugh, R. K., Cristofanilli, M., Martin, S. S., et al. (2016) Circulating cancer-associated macrophage-like cells differentiate malignant breast cancer and benign breast conditions, Cancer Epidemiol. Biomark. Prevent., 25, 1037-1042, https://doi.org/10.1158/1055-9965.epi-15-1221.
Kaigorodova, E. V., Zavaruev, I. S., Grishchenko, M. Yu., and Chernyshova, A. L. (2021) Features of occurrence of atypical/hybrid forms of EpCam+CD45+ cells in patients with endometrial cancer, Adv. Mol. Oncol., 8, 50, https://doi.org/10.17650/2313-805X-2021-8-4-5-163.
Augustyn, A., Adams, D. L., He, J., Qiao, Y., Verma, V., et al. (2020) Giant circulating cancer-associated macrophage-like cells are associated with disease recurrence and survival in non-small cell lung cancer treated with chemoradiation and atezolizumab, Clin. Lung Cancer, 22, e451-e465, https://doi.org/10.1016/j.cllc.2020.06.016.
Gardner, K. P., Aldakkak, M., Tang, C. M., Tsai, S., and Adams, D. L. (2021) Circulating stromal cells in resectable pancreatic cancer correlates to pathological stage and predicts for poor clinical outcomes, NPJ Precis. Oncol., 5, 25, https://doi.org/10.1038/s41698-021-00161-8.
Ding, J., Jin, W., Chen, C., Shao, Z., and Wu, J. (2012) Tumor associated macrophage × cancer cell hybrids may acquire cancer stem cell properties in breast cancer, PLoS One, 7, e41942, https://doi.org/10.1371/journal.pone.0041942.
Walker, B. S., Sutton, T. L., Zarour, L., Hunter, J. G., Wood, S. G., et al. (2021) Circulating hybrid cells: A novel liquid biomarker of treatment response in gastrointestinal cancers, Ann. Surg. Oncol., 28, 8567-8578, https://doi.org/10.1245/s10434-021-10379-2.
Henn, T. E., Anderson, A. N., Hollett, Y. R., Sutton, T. L., Walker, B. S., et al. (2021) Circulating hybrid cells predict presence of occult nodal metastases in oral cavity carcinoma, Head Neck, 43, 2193-2201, https://doi.org/10.1002/hed.26692.
Zarour, L., Swain, J., Billingsley, K., Lopez, C., Vaccaro, G., et al. (2018) Use of circulating cancer cell hybrids to monitor treatment response to hepatic arterial infusion in patients with colorectal cancer metastatic to the liver, HPB, 20, S352, https://doi.org/10.1016/j.hpb.2018.06.2584.
Montoya Mira, J., Sapre, A. A., Walker, B. S., Alvarez, J. B., Gustafson, K. T., et al. (2021) Label-free enrichment of rare unconventional circulating neoplastic cells using a microfluidic dielectrophoretic sorting device, Commun. Biol., 4, 1130, https://doi.org/10.1038/s42003-021-02651-8.
Cavazzoni, E., Bugiantella, W., Graziosi, L., Franceschini, M. S., and Donini, A. (2013) Malignant ascites: pathophysiology and treatment, Int. J. Clin. Oncol., 18, 1-9, https://doi.org/10.1007/s10147-012-0396-6.
Melzer, C., von der Ohe, J., and Hass, R. (2018) In vitro fusion of normal and neoplastic breast epithelial cells with human mesenchymal stroma/stem cells partially involves tumor necrosis factor receptor signaling, Stem Cells, 36, 977-989, https://doi.org/10.1002/stem.2819.
Weiler, J., and Dittmar, T. (2019) Minocycline impairs TNF-α-induced cell fusion of M13SV1-Cre cells with MDA-MB-435-pFDR1 cells by suppressing NF-κB transcriptional activity and its induction of target-gene expression of fusion-relevant factors, Cell Commun. Signal., 17, 71, https://doi.org/10.1186/s12964-019-0384-9.
Mullooly, M., McGowan, P. M., Crown, J., and Duffy, M. J. (2016) The ADAMs family of proteases as targets for the treatment of cancer, Cancer Biol. Ther., 17, 870-880, https://doi.org/10.1080/15384047.2016.1177684.
Sedger, L. M., and McDermott, M. F. (2014) TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants – past, present and future, Cytokine Growth Factor Rev., 25, 453-472, https://doi.org/10.1016/j.cytogfr.2014.07.016.
Chang, W., Fa, H., Xiao, D., and Wang, J. (2020) Targeting phosphatidylserine for cancer therapy: Prospects and challenges, Theranostics, 10, 9214-9229, https://doi.org/10.7150/thno.45125.
Weiler, J., Mohr, M., Zänker, K. S., and Dittmar, T. (2018) Matrix metalloproteinase-9 (MMP9) is involved in the TNF-α-induced fusion of human M13SV1-Cre breast epithelial cells and human MDA-MB-435-pFDR1 cancer cells, Cell Commun. Signal., 16, 14, https://doi.org/10.1186/s12964-018-0226-1.
Noubissi, F. K., Harkness, T., Alexander, C. M., and Ogle, B. M. (2015) Apoptosis-induced cancer cell fusion: a mechanism of breast cancer metastasis, FASEB J., 29, 4036-4045, https://doi.org/10.1096/fj.15-271098.
Dittmar, T., and Zänker, K. S. (2015) Tissue regeneration in the chronically inflamed tumor environment: Implications for cell fusion driven tumor progression and therapy resistant tumor hybrid cells, Int. J. Mol. Sci., 16, 30362-30381, https://doi.org/10.3390/ijms161226240.
Li, G., Kikuchi, K., Radka, M., Abraham, J., Rubin, B. P., and Keller, C. (2013) IL-4 receptor blockade abrogates satellite cell: Rhabdomyosarcoma fusion and prevents tumor establishment, Stem Cells, 31, 2304-2312, https://doi.org/10.1002/stem.1491.
Fernandes, C., Prabhu, P., Juvale, K., Suares, D., and Yc, M. (2019) Cancer cell fusion: A potential target to tackle drug-resistant and metastatic cancer cells, Drug Discov. Today, 24, 1836-1844, https://doi.org/10.1016/j.drudis.2019.05.024.
Sieler, Weiler, J., and Dittmar, T. (2021) Cell–cell fusion and the roads to novel properties of tumor hybrid cells, Cells, 10, 1465, https://doi.org/10.3390/cells10061465.
Greene, J. M., Schneble, E. J., Jackson, D. O., Hale, D. F., Vreeland, T. J., et al. (2016) A phase I/IIa clinical trial in stage IV melanoma of an autologous tumor–dendritic cell fusion (dendritoma) vaccine with low dose interleukin-2, Cancer Immunol. Immunother., 65, 383-392, https://doi.org/10.1007/s00262-016-1809-6.
Liu, W., Zou, M., Liu, T., Zeng, J., Li, X., et al. (2019) Expandable immunotherapeutic nanoplatforms engineered from cytomembranes of hybrid cells derived from cancer and dendritic cells, Adv. Mater., 31, 1900499, https://doi.org/10.1002/adma.201900499.
Funding
This research was supported by the Russian Science Foundation (grant no. 19-75-30016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kaigorodova, E.V., Kozik, A.V., Zavaruev, I.S. et al. Hybrid/Atypical Forms of Circulating Tumor Cells: Current State of the Art. Biochemistry Moscow 87, 380–390 (2022). https://doi.org/10.1134/S0006297922040071
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922040071